Deck 16: Acid-Base Equilibria
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/139
العب
ملء الشاشة (f)
Deck 16: Acid-Base Equilibria
1
According to the Arrhenius concept,an acid is a substance that ________.
A)is capable of donating one or more H+
B)causes an increase in the concentration of H+ in aqueous solutions
C)can accept a pair of electrons to form a coordinate covalent bond
D)reacts with the solvent to form the cation formed by autoionization of that solvent
E)tastes bitter
A)is capable of donating one or more H+
B)causes an increase in the concentration of H+ in aqueous solutions
C)can accept a pair of electrons to form a coordinate covalent bond
D)reacts with the solvent to form the cation formed by autoionization of that solvent
E)tastes bitter
causes an increase in the concentration of H+ in aqueous solutions
2
The molar concentration of hydronium ion in pure water at 25 °C is ________.
A)0.00
B)1.0 × 10-7
C)1.0 × 10-14
D)1.00
E)7.00
A)0.00
B)1.0 × 10-7
C)1.0 × 10-14
D)1.00
E)7.00
1.0 × 10-7
3
Which one of the following statements regarding Kw is false?
A)pKw is 14.00 at 25 °C.
B)The value of Kw is always 1.0 × 10-14.
C)Kw changes with temperature.
D)The value of Kw shows that water is a weak acid.
E)Kw is known as the ion product of water.
A)pKw is 14.00 at 25 °C.
B)The value of Kw is always 1.0 × 10-14.
C)Kw changes with temperature.
D)The value of Kw shows that water is a weak acid.
E)Kw is known as the ion product of water.
The value of Kw is always 1.0 × 10-14.
4
Of the acids in the table below,________ is the strongest acid. 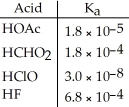
A)HOAc
B)HCHO2
C)HClO
D)HF
E)HOAc and HCHO2

A)HOAc
B)HCHO2
C)HClO
D)HF
E)HOAc and HCHO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
5
The hydride ion, 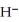 ,is a stronger base than the hydroxide ion,O
,is a stronger base than the hydroxide ion,O 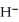 .The product(s)of the reaction of hydride ion with water is/are ________.
.The product(s)of the reaction of hydride ion with water is/are ________.
A)H3O+ (aq)
B)OH- (aq)+ H2 (g)
C)OH- (aq)+ 2H+ (aq)
D)no reaction occurs
E)H2O2 (aq)
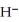 ,is a stronger base than the hydroxide ion,O
,is a stronger base than the hydroxide ion,O 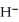 .The product(s)of the reaction of hydride ion with water is/are ________.
.The product(s)of the reaction of hydride ion with water is/are ________.A)H3O+ (aq)
B)OH- (aq)+ H2 (g)
C)OH- (aq)+ 2H+ (aq)
D)no reaction occurs
E)H2O2 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
6
Of the following acids,________ is a strong acid.
A)HNO2
B)H2CO3
C)HNO3
D)HClO
E)HF
A)HNO2
B)H2CO3
C)HNO3
D)HClO
E)HF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
7
The Ka of hypochlorous acid (HClO)is 3.0 × 10-8 at 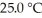 .What is the percent ionization of hypochlorous acid in a
.What is the percent ionization of hypochlorous acid in a 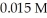 aqueous solution of HClO at
aqueous solution of HClO at 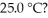
A)4.5 × 10-8
B)14
C)2.1 × 10-5
D)0.14
E)1.4 × 10-3
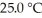 .What is the percent ionization of hypochlorous acid in a
.What is the percent ionization of hypochlorous acid in a 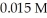 aqueous solution of HClO at
aqueous solution of HClO at 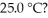
A)4.5 × 10-8
B)14
C)2.1 × 10-5
D)0.14
E)1.4 × 10-3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
8
The molar concentration of hydroxide ion in pure water at 25 °C is ________.
A)1.00
B)0.00
C)1.0 × 10-14
D)1.0 × 10-7
E)7.00
A)1.00
B)0.00
C)1.0 × 10-14
D)1.0 × 10-7
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
9
The magnitude of Kw indicates that ________.
A)water autoionizes very slowly
B)water autoionizes very quickly
C)water autoionizes only to a very small extent
D)the autoionization of water is exothermic
A)water autoionizes very slowly
B)water autoionizes very quickly
C)water autoionizes only to a very small extent
D)the autoionization of water is exothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
10
Classify the following compounds as weak acids (W)or strong acids (S): benzoic acid nitric acid acetic acid
A)W W W
B)S S S
C)S W W
D)W S S
E)W S W
A)W W W
B)S S S
C)S W W
D)W S S
E)W S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which one of the following is a Br∅nsted-Lowry acid?
A)(CH3)3NH+
B)CH3COOH
C)HF
D)HNO2
E)all of the above
A)(CH3)3NH+
B)CH3COOH
C)HF
D)HNO2
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which one of the following is the weakest acid?
A)HF (Ka = 6.8 × 10-4)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HCN (Ka = 4.9 × 10-10)
E)Acetic acid (Ka = 1.8 × 10-5)
A)HF (Ka = 6.8 × 10-4)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HCN (Ka = 4.9 × 10-10)
E)Acetic acid (Ka = 1.8 × 10-5)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
13
A Br∅nsted-Lowry base is defined as a substance that ________.
A)increases [H+] when placed in H2O
B)decreases [H+] when placed in H2O
C)increases [OH-] when placed in H2O
D)acts as a proton acceptor
E)acts as a proton donor
A)increases [H+] when placed in H2O
B)decreases [H+] when placed in H2O
C)increases [OH-] when placed in H2O
D)acts as a proton acceptor
E)acts as a proton donor

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which one of the following is a Br∅nsted-Lowry base?
A)(CH3)3N
B)CH3COOH
C)HF
D)HNO2
E)none of the above
A)(CH3)3N
B)CH3COOH
C)HF
D)HNO2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
15
A substance that is capable of acting as both an acid and as a base is ________.
A)autosomal
B)conjugated
C)amphiprotic
D)saturated
E)miscible
A)autosomal
B)conjugated
C)amphiprotic
D)saturated
E)miscible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
16
A Br∅nsted-Lowry acid is defined as a substance that ________.
A)increases Ka when placed in H2O
B)decreases [H+] when placed in H2O
C)increases [OH-] when placed in H2O
D)acts as a proton acceptor
E)acts as a proton donor
A)increases Ka when placed in H2O
B)decreases [H+] when placed in H2O
C)increases [OH-] when placed in H2O
D)acts as a proton acceptor
E)acts as a proton donor

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
17
Of the following acids,________ is not a strong acid.
A)HNO2
B)H2SO4
C)HNO3
D)HClO4
E)HCl
A)HNO2
B)H2SO4
C)HNO3
D)HClO4
E)HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
18
Classify the following compounds as weak acids (W)or strong acids (S): hydrocyanic acid hydrofluoric acid hydrobromic acid
A)S W W
B)S S S
C)W W S
D)W S S
E)W S W
A)S W W
B)S S S
C)W W S
D)W S S
E)W S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
19
Of the following,________ is a weak acid.
A)HF
B)HCl
C)HBr
D)HNO3
E)HClO4
A)HF
B)HCl
C)HBr
D)HNO3
E)HClO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
20
Classify the following compounds as weak acids (W)or strong acids (S): hydrobromic acid hydrochloric acid hydrofluoric acid
A)W W W
B)S S S
C)S W W
D)W S S
E)S S W
A)W W W
B)S S S
C)S W W
D)W S S
E)S S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following aqueous solutions has the highest [OH-]?
A)a solution with a pH of 3.0
B)a 1 × 10-4 M solution of HN![<strong>Which of the following aqueous solutions has the highest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_772c_9a0a_0566d18c9222_TB1194_11.jpg)
C)a solution with a pOH of 12.0
D)pure water
E)a 1 × 10-3 M solution of N![<strong>Which of the following aqueous solutions has the highest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_772d_9a0a_a3306cd2ca1a_TB1194_11.jpg) Cl
Cl
A)a solution with a pH of 3.0
B)a 1 × 10-4 M solution of HN
![<strong>Which of the following aqueous solutions has the highest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_772c_9a0a_0566d18c9222_TB1194_11.jpg)
C)a solution with a pOH of 12.0
D)pure water
E)a 1 × 10-3 M solution of N
![<strong>Which of the following aqueous solutions has the highest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_772d_9a0a_a3306cd2ca1a_TB1194_11.jpg) Cl
Cl
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following aqueous solutions has the lowest [OH-]?
A)a solution with a pH of 3.0
B)a 1 × 10-4 M solution of HN![<strong>Which of the following aqueous solutions has the lowest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_9e3e_9a0a_f93a559126d4_TB1194_11.jpg)
C)a solution with a pOH of 12.0
D)pure water
E)a 1 × 10-3 M solution of N![<strong>Which of the following aqueous solutions has the lowest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_9e3f_9a0a_2d706d411f94_TB1194_11.jpg) Cl
Cl
A)a solution with a pH of 3.0
B)a 1 × 10-4 M solution of HN
![<strong>Which of the following aqueous solutions has the lowest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_9e3e_9a0a_f93a559126d4_TB1194_11.jpg)
C)a solution with a pOH of 12.0
D)pure water
E)a 1 × 10-3 M solution of N
![<strong>Which of the following aqueous solutions has the lowest [OH<sup>-</sup>]?</strong> A)a solution with a pH of 3.0 B)a 1 × 10<sup>-4</sup> M solution of HN C)a solution with a pOH of 12.0 D)pure water E)a 1 × 10<sup>-3</sup> M solution of N Cl](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615b_9e3f_9a0a_2d706d411f94_TB1194_11.jpg) Cl
Cl
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
23
Using the data in the table,which of the conjugate acids below is the strongest acid? 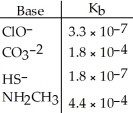
A)HClO
B)HCO3-
C)H2S
D)NH3CH3+
E)H2S and HClO

A)HClO
B)HCO3-
C)H2S
D)NH3CH3+
E)H2S and HClO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
24
Using the data in the table,which of the conjugate bases below is the weakest base? 
A)OAc-
B)C7H5O2-
C)NO2-
D)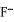
E)OAc- and C7H5O2-

A)OAc-
B)C7H5O2-
C)NO2-
D)
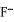
E)OAc- and C7H5O2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
25
Classify the following compounds as weak bases (W)or strong bases (S): methylamine carbonate ion potassium ion
A)S S S
B)S W W
C)W S S
D)W W W
E)W S W
A)S S S
B)S W W
C)W S S
D)W W W
E)W S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
26
Using the data in the table,which of the conjugate acids below is the weakest acid? 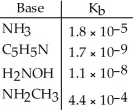
A)NH4+
B)C5H5NH+
C)H3NOH+
D)NH3CH3+
E)NH4+ and NH3CH3+

A)NH4+
B)C5H5NH+
C)H3NOH+
D)NH3CH3+
E)NH4+ and NH3CH3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
27
Using the data in the table,which of the conjugate acids below is the weakest acid? 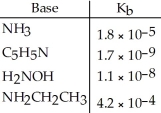
A)NH4+
B)C5H5NH+
C)NH3CH2CH3+
D)H3NOH+
E)NH4+ and NH3CH3+

A)NH4+
B)C5H5NH+
C)NH3CH2CH3+
D)H3NOH+
E)NH4+ and NH3CH3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following ions will act as a strong base in water?
A)HS-
B)F-
C)NO2-
D)ClO-
E)None of the above will act as a strong base in water.
A)HS-
B)F-
C)NO2-
D)ClO-
E)None of the above will act as a strong base in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
29
Using the data in the table,which of the conjugate bases below is the strongest base? 
A)OAc-
B)C7H5O2-
C)NO2-
D)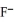
E)OAc- and C7H5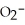

A)OAc-
B)C7H5O2-
C)NO2-
D)
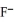
E)OAc- and C7H5
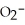

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
30
An aqueous solution of a particular compound has pH = 7.46.The compound is ________.
A)a weak base
B)a weak acid
C)a strong acid
D)a strong base
E)a salt
A)a weak base
B)a weak acid
C)a strong acid
D)a strong base
E)a salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
31
Using the data in the table,which of the conjugate acids below is the strongest acid? 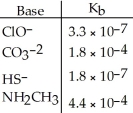
A)HClO
B)HCO3-
C)H2S
D)NH3CH3+
E)H2S and HClO

A)HClO
B)HCO3-
C)H2S
D)NH3CH3+
E)H2S and HClO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
32
Using the data in the table,which of the conjugate bases below is the weakest base? 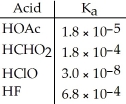
A)OAc-
B)CHO2-
C)ClO-
D)F-
E)OAc- and CHO2-

A)OAc-
B)CHO2-
C)ClO-
D)F-
E)OAc- and CHO2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
33
Using the data in the table,which of the conjugate bases below is the weakest base? 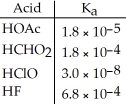
A)OAc-
B)CHO2-
C)ClO-
D)F-
E)OAc- and CHO2-

A)OAc-
B)CHO2-
C)ClO-
D)F-
E)OAc- and CHO2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
34
Of the following substances,an aqueous solution of ________ will form basic solutions. NaHS Cu(NO3)2 KHCO3 NaF
A)NaHS, Cu(NO3)2
B)KHCO3, NaHS
C)NaF only
D)NaF, KHCO3
E)NaHS, KHCO3 and NaF
A)NaHS, Cu(NO3)2
B)KHCO3, NaHS
C)NaF only
D)NaF, KHCO3
E)NaHS, KHCO3 and NaF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
35
Ammonia is a ________.
A)weak acid
B)strong base
C)weak base
D)strong acid
E)salt
A)weak acid
B)strong base
C)weak base
D)strong acid
E)salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
36
A- is a weak base.Which equilibrium corresponds to the equilibrium constant Ka for HA?
A)HA (aq)+ H2O (l) H2A+ (aq)+ OH- (aq)
H2A+ (aq)+ OH- (aq)
B)A- (aq)+ H3O+ (aq) HA (aq)+ H2O (l)
HA (aq)+ H2O (l)
C)HA (aq)+ H2O (l) H3O+ (aq)+ A- (aq)
H3O+ (aq)+ A- (aq)
D)A- (aq)+ H2O (l) HA (aq)+ OH- (aq)
HA (aq)+ OH- (aq)
E)A- (aq)+ OH- (aq) HOA2- (aq)
HOA2- (aq)
A)HA (aq)+ H2O (l)
 H2A+ (aq)+ OH- (aq)
H2A+ (aq)+ OH- (aq)B)A- (aq)+ H3O+ (aq)
 HA (aq)+ H2O (l)
HA (aq)+ H2O (l)C)HA (aq)+ H2O (l)
 H3O+ (aq)+ A- (aq)
H3O+ (aq)+ A- (aq)D)A- (aq)+ H2O (l)
 HA (aq)+ OH- (aq)
HA (aq)+ OH- (aq)E)A- (aq)+ OH- (aq)
 HOA2- (aq)
HOA2- (aq)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following ions will act as a weak base in water?
A)OH-
B)Cl-
C)NO3-
D)ClO-
E)None of the above will act as a weak base in water.
A)OH-
B)Cl-
C)NO3-
D)ClO-
E)None of the above will act as a weak base in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
38
Classify the following compounds as weak bases (W)or strong bases (S): ammonia fluoride ion sodium ion
A)S S S
B)S W W
C)W W W
D)W S S
E)W S W
A)S S S
B)S W W
C)W W W
D)W S S
E)W S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
39
Classify the following compounds as weak acids (W)or strong acids (S): nitric acid perchloric acid chloric acid
A)W W W
B)W S S
C)S S S
D)S W W
E)W S W
A)W W W
B)W S S
C)S S S
D)S W W
E)W S W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
40
HA is a weak acid.Which equilibrium corresponds to the equilibrium constant Kb for A-?
A)HA (aq)+ H2O (l) H2A+ (aq)+ OH-(aq)
H2A+ (aq)+ OH-(aq)
B)A- (aq)+ H3O+ (aq) HA (aq)+ H2O (l)
HA (aq)+ H2O (l)
C)HA (aq)+ OH- (aq) H2O (l)+ H+ (aq)
H2O (l)+ H+ (aq)
D)A- (aq)+ H2O (l) HA (aq)+ OH- (aq)
HA (aq)+ OH- (aq)
E)A- (aq)+ OH- (aq) HOA2- (aq)
HOA2- (aq)
A)HA (aq)+ H2O (l)
 H2A+ (aq)+ OH-(aq)
H2A+ (aq)+ OH-(aq)B)A- (aq)+ H3O+ (aq)
 HA (aq)+ H2O (l)
HA (aq)+ H2O (l)C)HA (aq)+ OH- (aq)
 H2O (l)+ H+ (aq)
H2O (l)+ H+ (aq)D)A- (aq)+ H2O (l)
 HA (aq)+ OH- (aq)
HA (aq)+ OH- (aq)E)A- (aq)+ OH- (aq)
 HOA2- (aq)
HOA2- (aq)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the pH of an aqueous solution at 25.0 °C that contains 1.35 × 10-8 M hydroxide ion?
A)7.87
B)8.00
C)6.13
D)1.35
E)7.00
A)7.87
B)8.00
C)6.13
D)1.35
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
42
The conjugate base of CH3NH3+ is ________.
A)CH3NH2+
B)CH3NH2-
C)CH3NH+
D)CH3NH2
E)none of the above
A)CH3NH2+
B)CH3NH2-
C)CH3NH+
D)CH3NH2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the conjugate acid of HCO3-?
A)CO22-
B)H2CO3
C)HCO22-
D)CO32-
E)none of the above
A)CO22-
B)H2CO3
C)HCO22-
D)CO32-
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
44
Calculate the pH of a solution at 25.0 °C that contains 2.95 × 10-12 M hydronium ions.
A)2.95
B)11.53
C)7.00
D)12.00
E)2.47
A)2.95
B)11.53
C)7.00
D)12.00
E)2.47

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
45
Of the following substances,an aqueous solution of ________ will form basic solutions. NH4Br Pb(NO3)2 K2CO3 NaF
A)NH4Br, Pb(NO3)2
B)K2CO3, NH4Br
C)NaF only
D)NaF, K2CO3
E)NH4Br only
A)NH4Br, Pb(NO3)2
B)K2CO3, NH4Br
C)NaF only
D)NaF, K2CO3
E)NH4Br only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
46
The conjugate base of HSO4- is ________.
A)H2SO4
B)HSO4+
C)H+
D)SO42-
E)HSO3+
A)H2SO4
B)HSO4+
C)H+
D)SO42-
E)HSO3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
47
In the gas phase reaction below,NH3 is acting as a(n)________. 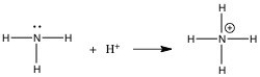
A)Br∅nsted-Lowry acid
B)Br∅nsted-Lowry base
C)Lewis base
D)Lewis acid
E)Arrhenius acid

A)Br∅nsted-Lowry acid
B)Br∅nsted-Lowry base
C)Lewis base
D)Lewis acid
E)Arrhenius acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
48
A 0.5 M solution of ________ has a pH of 7.0.
A)K2S
B)KF
C)KNO3
D)NH4Br
E)NaF
A)K2S
B)KF
C)KNO3
D)NH4Br
E)NaF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
49
The conjugate acid of SO42- is ________.
A)OH-
B)H2SO4
C)HSO4-
D)HSO42-
E)H3SO4+
A)OH-
B)H2SO4
C)HSO4-
D)HSO42-
E)H3SO4+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the conjugate acid of OH-?
A)O2
B)H2O
C)O-
D)O2-
E)H3O+
A)O2
B)H2O
C)O-
D)O2-
E)H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the pH of an aqueous solution at 25.0 °C that contains 2.50 × 10-4 M hydronium ion?
A)10.4
B)4.00
C)2.50
D)3.60
E)7.00
A)10.4
B)4.00
C)2.50
D)3.60
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
52
Calculate the concentration (in M)of hydronium ions in a solution at 25.0 °C with a pOH of 3.58.
A)2.63 × 10-4
B)2.63 × 1010
C)3.80 × 10-11
D)3.80 × 103
E)1.00 × 10-7
A)2.63 × 10-4
B)2.63 × 1010
C)3.80 × 10-11
D)3.80 × 103
E)1.00 × 10-7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the conjugate acid of NH2-?
A)NH2+
B)NH3+
C)NH4+
D)NH3
E)NH4OH
A)NH2+
B)NH3+
C)NH4+
D)NH3
E)NH4OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
54
What is the pOH of an aqueous solution at 25.0 °C that contains 1.35 × 10-8 M hydroxide ion?
A)6.13
B)7.87
C)1.35
D)8.00
E)7.00
A)6.13
B)7.87
C)1.35
D)8.00
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
55
Of the compounds below,a 0.1 M aqueous solution of ________ will have the highest pH.
A)KCN, Ka of HCN = 4.0 × 10-10
B)NH4NO3, Kb of NH3 = 1.8 × 10-5
C)NaOAc, Ka of HOAc = 1.8 × 10-5
D)NaClO, Ka of HClO = 3.2 × 10-8
E)NaHS, Kb of HS- = 1.8 × 10-7
A)KCN, Ka of HCN = 4.0 × 10-10
B)NH4NO3, Kb of NH3 = 1.8 × 10-5
C)NaOAc, Ka of HOAc = 1.8 × 10-5
D)NaClO, Ka of HClO = 3.2 × 10-8
E)NaHS, Kb of HS- = 1.8 × 10-7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the pOH of an aqueous solution at 25.0 °C that contains 2.50 × 10-4 M hydronium ion?
A)3.60
B)4.00
C)2.50
D)10.4
E)7.00
A)3.60
B)4.00
C)2.50
D)10.4
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
57
Calculate the pOH of a solution at 25.0 °C that contains 2.95 × 10-12 M hydronium ions.
A)12.00
B)2.95
C)7.00
D)2.47
E)11.53
A)12.00
B)2.95
C)7.00
D)2.47
E)11.53

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following acids will be the strongest?
A)H2SO4
B)HSO4-
C)H2SO3
D)H2SeO4
E)HSO3-
A)H2SO4
B)HSO4-
C)H2SO3
D)H2SeO4
E)HSO3-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
59
Of the following,which is the strongest acid?
A)HClO
B)HClO3
C)HClO2
D)HClO4
E)HIO
A)HClO
B)HClO3
C)HClO2
D)HClO4
E)HIO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
60
The conjugate base of HPO42- is ________.
A)PO43-
B)H2PO4
C)H3PO4
D)H2PO4-
E)none of the above
A)PO43-
B)H2PO4
C)H3PO4
D)H2PO4-
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
61
An aqueous solution of NaF is prepared by dissolving 0.350 mol of NaF in sufficient water to yield 1.0 L of solution.The pH of the solution was 8.93 at 25.0 °C.The Kb of F- is ________.
A)1.2 × 10-5
B)2.1 × 10-10
C)6.9 × 10-9
D)2.8 × 10-12
E)9.9 × 10-2
A)1.2 × 10-5
B)2.1 × 10-10
C)6.9 × 10-9
D)2.8 × 10-12
E)9.9 × 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
62
The pH of a 0.60 M aqueous solution of formic acid,HCHO2,at 25.0 °C is 1.98.What is the value of Ka for formic acid?
A)2.0 × 10-5
B)1.8 × 10-4
C)6.0 × 10-5
D)3.5 × 10-4
E)none of the above
A)2.0 × 10-5
B)1.8 × 10-4
C)6.0 × 10-5
D)3.5 × 10-4
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
63
An aqueous solution contains 0.500 M NaOH at 25.0 °C.The pH of the solution is ________.
A)0.500
B)13.70
C)0.301
D)7.00
E)13.50
A)0.500
B)13.70
C)0.301
D)7.00
E)13.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
64
An aqueous basic solution has a concentration of 0.050 M and Kb is 4.4 × 10-4.What is the concentration of hydronium ion in this solution (M)?
A)2.2 × 10-13
B)2.2 × 10-12
C)2.9 × 10-13
D)0.050
E)4.5 × 10-13
A)2.2 × 10-13
B)2.2 × 10-12
C)2.9 × 10-13
D)0.050
E)4.5 × 10-13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
65
The Ka of hypochlorous acid (HClO)is 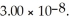 What is the pH at 25.0 °C of an aqueous solution that is
What is the pH at 25.0 °C of an aqueous solution that is 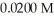 in
in 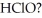
A)+2.45
B)-2.45
C)-9.22
D)+9.22
E)+4.61
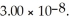 What is the pH at 25.0 °C of an aqueous solution that is
What is the pH at 25.0 °C of an aqueous solution that is 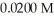 in
in 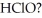
A)+2.45
B)-2.45
C)-9.22
D)+9.22
E)+4.61

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
66
HZ is a weak acid.An aqueous solution of HZ is prepared by dissolving 0.020 mol of HZ in sufficient water to yield 1.0 L of solution.The pH of the solution was 4.93 at 25.0 °C.The Ka of HZ is ________.
A)1.2 × 10-5
B)6.9 × 10-9
C)1.4 × 10-10
D)9.9 × 10-2
E)2.8 × 10-12
A)1.2 × 10-5
B)6.9 × 10-9
C)1.4 × 10-10
D)9.9 × 10-2
E)2.8 × 10-12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
67
Calculate the concentration (in M)of hydroxide ions in a solution at 25.0 °C with a pOH of 3.58.
A)2.63 × 1010
B)3.80 × 10-11
C)1.00 × 10-7
D)3.80 × 103
E)2.63 × 10-4
A)2.63 × 1010
B)3.80 × 10-11
C)1.00 × 10-7
D)3.80 × 103
E)2.63 × 10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
68
The Ka of acetic acid (HC2H3O2)is 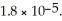 What is the pH at 25.0 °C of an aqueous solution that is
What is the pH at 25.0 °C of an aqueous solution that is 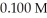 in
in 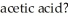
A)+2.87
B)-2.87
C)-11.13
D)+11.13
E)+6.61
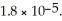 What is the pH at 25.0 °C of an aqueous solution that is
What is the pH at 25.0 °C of an aqueous solution that is 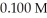 in
in 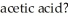
A)+2.87
B)-2.87
C)-11.13
D)+11.13
E)+6.61

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
69
The pH of a 0.25 M aqueous solution of hydrofluoric acid,HF,at 25.0 °C is 2.03.What is the value of Ka for HF?
A)2.0 × 10-9
B)1.1 × 10-9
C)6.0 × 10-5
D)3.5 × 10-4
E)none of the above
A)2.0 × 10-9
B)1.1 × 10-9
C)6.0 × 10-5
D)3.5 × 10-4
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
70
The pH of a 0.55 M aqueous solution of hypobromous acid,HBrO,at 25.0 °C is 4.48.What is the value of Ka for HBrO?
A)2.0 × 10-9
B)1.1 × 10-9
C)6.0 × 10-5
D)3.3 × 10-5
E)3.0 × 104
A)2.0 × 10-9
B)1.1 × 10-9
C)6.0 × 10-5
D)3.3 × 10-5
E)3.0 × 104

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
71
A 0.10 M aqueous solution of the weak base B at 25.0 °C has a pH of 8.00.The value of Kb for B is ________.
A)1.0 × 10-15
B)1.0 × 10-11
C)1.0 × 10-6
D)1.0 × 10-12
E)none of the above
A)1.0 × 10-15
B)1.0 × 10-11
C)1.0 × 10-6
D)1.0 × 10-12
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
72
The pOH of a 0.10 M solution of a weak base is 4.18.What is the Kb for this base?
A)8.8 × 10-8
B)2.1 × 10-4
C)6.6 × 10-4
D)4.4 × 10-8
E)2.0 × 10-5
A)8.8 × 10-8
B)2.1 × 10-4
C)6.6 × 10-4
D)4.4 × 10-8
E)2.0 × 10-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
73
The pH of a 0.25 M aqueous solution ammonia,NH3,at 25.0 °C is 9.50.What is the value of Kb for NH3?
A)2.5 × 10-1
B)4.0 × 10-19
C)4.0 × 10-9
D)3.2 × 10-5
E)none of the above
A)2.5 × 10-1
B)4.0 × 10-19
C)4.0 × 10-9
D)3.2 × 10-5
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
74
The acid-dissociation constants of sulfurous acid (H2SO3)are Ka1 = 1.7 × 10-2 and 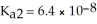 at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.
at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.
A)4.53
B)1.28
C)1.86
D)6.21
E)1.93
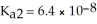 at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.
at 25.0 °C.Calculate the pH of a 0.163 M aqueous solution of sulfurous acid.A)4.53
B)1.28
C)1.86
D)6.21
E)1.93

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
75
Determine the pH of a 0.35 M aqueous solution of CH3NH2 (methylamine).The Kb of methylamine is 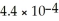 .
.
A)10.00
B)3.86
C)12.09
D)1.96
E)13.24
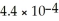 .
.A)10.00
B)3.86
C)12.09
D)1.96
E)13.24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
76
A 0.14 M aqueous solution of the weak acid HA at 25.0 °C has a pH of 3.15.The value of Ka for HA is ________.
A)7.08 × 10-4
B)3.58 × 10-6
C)5.01 × 10-7
D)7.02 × 10-8
E)none of the above
A)7.08 × 10-4
B)3.58 × 10-6
C)5.01 × 10-7
D)7.02 × 10-8
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
77
An aqueous solution contains 0.390 M HCl at 25.0 °C.The pH of the solution is ________.
A)0.41
B)0.390
C)13.61
D)13.59
E)7.00
A)0.41
B)0.390
C)13.61
D)13.59
E)7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
78
An aqueous basic solution has a concentration of 0.050 M and Kb is 4.4 × 10-4.What is the concentration of hydroxide ion in this solution (M)?
A)2.2 × 10-5
B)4.5 × 10-3
C)2.9 × 10-3
D)4.7 × 10-3
E)0.050
A)2.2 × 10-5
B)4.5 × 10-3
C)2.9 × 10-3
D)4.7 × 10-3
E)0.050

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
79
The acid-dissociation constants of phosphoric acid (H3PO4)are 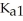 = 7.5 × 10-3,
= 7.5 × 10-3, 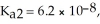 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
A)1.82
B)0.40
C)2.51
D)0.86
E)0.13
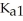 = 7.5 × 10-3,
= 7.5 × 10-3, 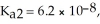 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?A)1.82
B)0.40
C)2.51
D)0.86
E)0.13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck
80
A 0.22 M aqueous solution of the weak acid HA at 25.0 °C has a pH of 4.15.The value of Ka for HA is ________.
A)7.1 × 10-5
B)3.2 × 10-4
C)2.2 × 10-1
D)2.3 × 10-8
E)3.2 × 104
A)7.1 × 10-5
B)3.2 × 10-4
C)2.2 × 10-1
D)2.3 × 10-8
E)3.2 × 104

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 139 في هذه المجموعة.
فتح الحزمة
k this deck








