Deck 21: Nuclear Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/178
العب
ملء الشاشة (f)
Deck 21: Nuclear Chemistry
1
Which one of the following is a correct representation of a beta particle?
A)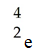
B)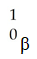
C)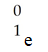
D)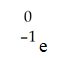
E)
A)
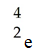
B)
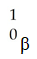
C)
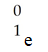
D)
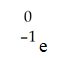
E)

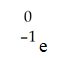
2
What is the atomic number of a neutron?
A)3
B)1
C)2
D)0
E)4
A)3
B)1
C)2
D)0
E)4
0
3
Which one of the following processes results in an increase in the atomic number?
A)gamma emission
B)positron emission
C)beta emission
D)alpha emission
E)corrosion
A)gamma emission
B)positron emission
C)beta emission
D)alpha emission
E)corrosion
beta emission
4
Alpha decay produces a new nucleus whose ________ than those respectively of the original nucleus.
A)atomic number is 2 less and mass number is 2 less
B)atomic number is 1 less and mass number is 2 less
C)atomic number is 2 less and mass number is 4 less
D)atomic number is 2 more and mass number is 4 more
E)atomic number is 2 more and mass number is 2 less
A)atomic number is 2 less and mass number is 2 less
B)atomic number is 1 less and mass number is 2 less
C)atomic number is 2 less and mass number is 4 less
D)atomic number is 2 more and mass number is 4 more
E)atomic number is 2 more and mass number is 2 less

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which type of radioactive decay results in no change in mass number and atomic number for the starting nucleus?
A)alpha
B)beta
C)positron emission
D)electron capture
E)gamma
A)alpha
B)beta
C)positron emission
D)electron capture
E)gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which one of the following is a correct representation of an alpha particle?
A)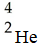
B)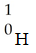
C)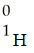
D)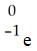
E)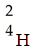
A)
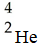
B)
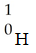
C)
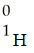
D)
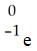
E)
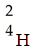

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
7
The formation of krypton from rubidium decay is a result of ________.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
8
Atoms containing radioactive nuclei are called ________.
A)radionuclides
B)radioisotopes
C)nucleons
D)nuclides
E)radioisophores
A)radionuclides
B)radioisotopes
C)nucleons
D)nuclides
E)radioisophores

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following is a correct representation of a positron?
A)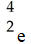
B)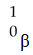
C)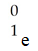
D)
E)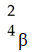
A)
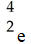
B)
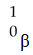
C)
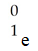
D)

E)
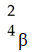

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
10
Atoms with the same atomic number and different mass numbers ________.
A)do not exist
B)are isomers
C)are isotopes
D)are allotropes
E)are resonance structures
A)do not exist
B)are isomers
C)are isotopes
D)are allotropes
E)are resonance structures

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
11
What radioactive element is used to diagnose medical conditions of the heart and arteries?
A)cobalt-60
B)thallium-201
C)radium-226
D)radon-222
E)thorium-234
A)cobalt-60
B)thallium-201
C)radium-226
D)radon-222
E)thorium-234

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
12
What happens to the mass number and the atomic number of an element when it emits gamma radiation?
A)The mass number remains unchanged while the atomic number decreases by one.
B)The mass number decreases by four and the atomic number decreases by two.
C)The mass number increases by four and the atomic number increases by two.
D)The mass number remains unchanged while the atomic number increases by one.
E)The mass number and atomic numbers remain unchanged.
A)The mass number remains unchanged while the atomic number decreases by one.
B)The mass number decreases by four and the atomic number decreases by two.
C)The mass number increases by four and the atomic number increases by two.
D)The mass number remains unchanged while the atomic number increases by one.
E)The mass number and atomic numbers remain unchanged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many radioactive decay series exist in nature?
A)0
B)1
C)2
D)3
E)10
A)0
B)1
C)2
D)3
E)10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
14
Of the following processes,which one changes the atomic number?
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
E)All of these processes change the atomic numbers.
A)alpha emission
B)beta emission
C)electron capture
D)positron emission
E)All of these processes change the atomic numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
15
Carbon-11 decays by ________.
A)alpha emission
B)beta emission
C)positron emission
D)photon emission
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)photon emission
E)neutron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
16
In what type of radioactive decay does the atomic number of the product increase by one?
A)alpha
B)beta
C)gamma
D)positron emission
E)electron capture
A)alpha
B)beta
C)gamma
D)positron emission
E)electron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the missing product from this reaction?
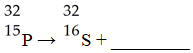
A)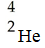
B)
C)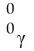
D)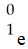
E)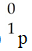
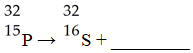
A)
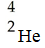
B)

C)
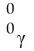
D)
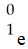
E)
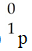

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
18
All atoms of a given element have the same ________.
A)mass number
B)number of nucleons
C)atomic mass
D)number of neutrons
E)atomic number
A)mass number
B)number of nucleons
C)atomic mass
D)number of neutrons
E)atomic number

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
19
At approximately what number of protons,or neutrons,does the 1:1 ratio of protons to neutrons start to produce unstable nuclei?
A)10
B)20
C)30
D)50
E)80
A)10
B)20
C)30
D)50
E)80

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
20
What happens to the mass number and the atomic number of an element when it undergoes beta decay?
A)Neither the mass number nor the atomic number changes.
B)The mass number decreases by 4 and the atomic number decreases by 2.
C)The mass number does not change and the atomic number increases by 1.
D)The mass number does not change and the atomic number decreases by 2.
E)The mass number increases by 2 and the atomic number increases by 1.
A)Neither the mass number nor the atomic number changes.
B)The mass number decreases by 4 and the atomic number decreases by 2.
C)The mass number does not change and the atomic number increases by 1.
D)The mass number does not change and the atomic number decreases by 2.
E)The mass number increases by 2 and the atomic number increases by 1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
21
The half-life of 218Po is 3.1 minutes.How much of a 155 gram sample remains after 0.40 hours?
A)0.00067 g
B)0.0072 g
C)0.72 g
D)0.0047 g
E)none of the above
A)0.00067 g
B)0.0072 g
C)0.72 g
D)0.0047 g
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
22
In the nuclear transmutation represented by
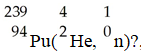 ,what is the product?
,what is the product?
A)uranium-242
B)curium-245
C)curium-242
D)uranium-245
E)uranium-243
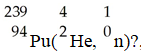 ,what is the product?
,what is the product?A)uranium-242
B)curium-245
C)curium-242
D)uranium-245
E)uranium-243

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
23
The belt of nuclear stability ends with the element ________.
A)lead
B)polonium
C)radon
D)astatine
E)bismuth
A)lead
B)polonium
C)radon
D)astatine
E)bismuth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
24
The product of the nuclear reaction in which 28Si is subjected to neutron capture followed by alpha emission is ________.
A).31S
B).33S
C).23Mg
D).25Mg
E).25Al
A).31S
B).33S
C).23Mg
D).25Mg
E).25Al

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
25
In the nuclear transmutation,
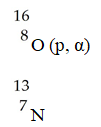 ,what is the bombarding particle?
,what is the bombarding particle?
A)an alpha particle
B)a beta particle
C)a gamma photon
D)a proton
E)a phosphorus nucleus
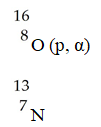 ,what is the bombarding particle?
,what is the bombarding particle?A)an alpha particle
B)a beta particle
C)a gamma photon
D)a proton
E)a phosphorus nucleus

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
26
The transmutation in which a curium-242 nucleus is bombarded with an alpha particle to produce a californium-245 nucleus is represented by ________.
A)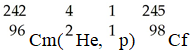
B)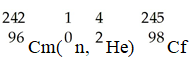
C)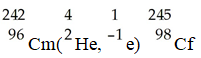
D)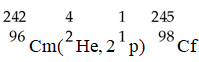
E)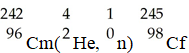
A)
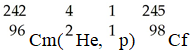
B)
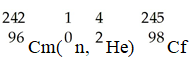
C)
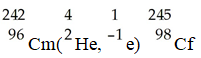
D)
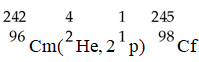
E)
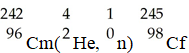

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
27
In the nuclear transmutation represented by
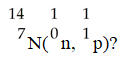 ,what is the emitted particle?
,what is the emitted particle?
A)neutron
B)proton
C)positron
D)alpha particle
E)electron
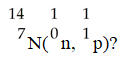 ,what is the emitted particle?
,what is the emitted particle?A)neutron
B)proton
C)positron
D)alpha particle
E)electron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is required for a nuclear transmutation to occur?
A)very high temperature
B)a corrosive environment
C)a particle to collide with a nucleus or neutron
D)spontaneous nuclear decay
E)gamma emission
A)very high temperature
B)a corrosive environment
C)a particle to collide with a nucleus or neutron
D)spontaneous nuclear decay
E)gamma emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which one of the following can be done to shorten the half-life of the radioactive decay of uranium-238?
A)freeze it
B)heat it
C)convert it to UF6
D)oxidize it to the +2 oxidation state
E)none of the above
A)freeze it
B)heat it
C)convert it to UF6
D)oxidize it to the +2 oxidation state
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
30
Cobalt-60 is produced by a three reaction process involving neutron capture,beta-emission,and neutron capture.The initial reactant in the production of cobalt-60 is ________.
A).59Co
B).56Fe
C).58Fe
D).61Co
E).60Fe
A).59Co
B).56Fe
C).58Fe
D).61Co
E).60Fe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
31
Transuranium elements have atomic numbers greater than ________.
A)90
B)91
C)92
D)93
E)94
A)90
B)91
C)92
D)93
E)94

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
32
The mode of decay of 32P is ________.
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture
A)alpha emission
B)beta emission
C)positron emission
D)electron capture
E)neutron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
33
Bombardment of uranium-238 with a deuteron (hydrogen-2)generates neptunium-237 and ________ neutrons.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
34
The beta decay of cesium-137 has a half-life of 30.0 years.How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg?
A)46
B)32
C)3.2
D)50
E)52
A)46
B)32
C)3.2
D)50
E)52

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
35
Cesium-131 has a half-life of 9.7 days.What percent of a cesium-131 sample remains after 60 days?
A)100
B)0
C)1.4
D)98.6
E)more information is needed to solve the problem
A)100
B)0
C)1.4
D)98.6
E)more information is needed to solve the problem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
36
The transmutation in which neptunium-239 is produced via bombardment of uranium-238 with a neutron is represented by ________.
A)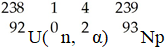
B)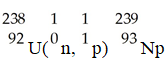
C)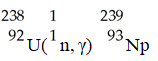
D)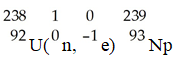
E)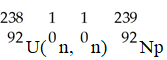
A)
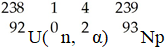
B)
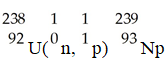
C)
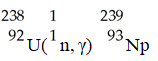
D)
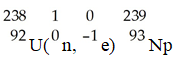
E)
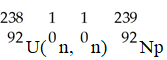

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the product for the nuclear transmutation represented by
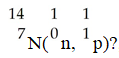
A)carbon-12
B)carbon-16
C)nitrogen-16
D)carbon-14
E)nitrogen-15
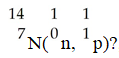
A)carbon-12
B)carbon-16
C)nitrogen-16
D)carbon-14
E)nitrogen-15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is emitted in the nuclear transmutation,
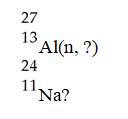
A)an alpha particle
B)a beta particle
C)a neutron
D)a proton
E)a gamma photon
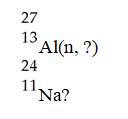
A)an alpha particle
B)a beta particle
C)a neutron
D)a proton
E)a gamma photon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of these nuclides is most likely to be radioactive?
A)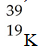
B)
C)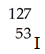
D)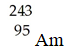
E)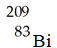
A)
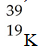
B)

C)
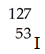
D)
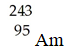
E)
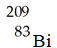

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which one of the following requires a particle accelerator to occur?
A)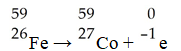
B)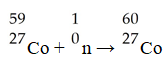
C)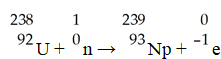
D)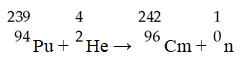
E)none of the above
A)
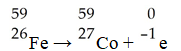
B)
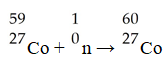
C)
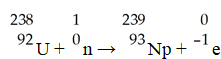
D)
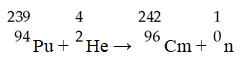
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which one of the following is used as a radiotracer to study blood?
A)iron-59
B)technetium-99
C)sodium-23
D)iodine-131
E)phosphorus-32
A)iron-59
B)technetium-99
C)sodium-23
D)iodine-131
E)phosphorus-32

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
42
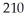 Pb has a half-life of 22.3 years and decays to produce 206Hg.If you start with 7.50 g of 210Pb,how many grams of 206Hg will you have after 17.5 years?
Pb has a half-life of 22.3 years and decays to produce 206Hg.If you start with 7.50 g of 210Pb,how many grams of 206Hg will you have after 17.5 years?A)4.35
B)3.15
C)3.09
D)0.0600
E)1.71

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
43
The carbon-14 dating method can be used to determine the age of a ________.
A)flint arrowhead
B)papyrus scroll
C)stone axe head
D)clay pot
E)rock
A)flint arrowhead
B)papyrus scroll
C)stone axe head
D)clay pot
E)rock

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
44
When two atoms of 2H are fused to form one atom of 4He,the total energy evolved is 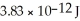 .What is the total change in mass (in kg)for this reaction?
.What is the total change in mass (in kg)for this reaction? 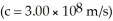
A)1.28 × 10-17
B)4.26 × 10-26
C)3.45 × 108
D)1.15
E)4.26 × 10-29
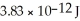 .What is the total change in mass (in kg)for this reaction?
.What is the total change in mass (in kg)for this reaction? 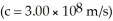
A)1.28 × 10-17
B)4.26 × 10-26
C)3.45 × 108
D)1.15
E)4.26 × 10-29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
45
The curie is a measure of the ________.
A)number of disintegrations per second of a radioactive substance
B)total energy absorbed by an object exposed to a radioactive source
C)lethal threshold for radiation exposure
D)number of alpha particles emitted by exactly one gram of a radioactive substance
E)None of the above is correct.
A)number of disintegrations per second of a radioactive substance
B)total energy absorbed by an object exposed to a radioactive source
C)lethal threshold for radiation exposure
D)number of alpha particles emitted by exactly one gram of a radioactive substance
E)None of the above is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the half-life (in min)of this radionuclide?
A)0.024
B)0.022
C)32
D)0.032
E)45
A)0.024
B)0.022
C)32
D)0.032
E)45

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
47
The mass of a proton is 1.673 × 10-24 g.The mass of a neutron is 1.675 × 10-24 g.The mass of the nucleus of an 56Fe atom is 9.289 × 10-23 g.What is the nuclear binding energy (in J)for a 56Fe nucleus? 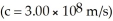
A)2.57 × 10-16
B)7.72 × 10-8
C)8.36 × 10-9
D)7.72 × 10-11
E)6.07 × 106
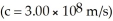
A)2.57 × 10-16
B)7.72 × 10-8
C)8.36 × 10-9
D)7.72 × 10-11
E)6.07 × 106

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
48
The half-life of 131I is 0.220 years.How much of a 500.0 mg sample remains after 24 hours?
A)496 mg
B)560 mg
C)219 mg
D)405 mg
E)337 mg
A)496 mg
B)560 mg
C)219 mg
D)405 mg
E)337 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
49
The basis for the carbon-14 dating method is that ________.
A)the amount of carbon-14 in all objects is the same
B)carbon-14 is very unstable and is readily lost from the atmosphere
C)the ratio of carbon-14 to carbon-12 in the atmosphere is a constant
D)living tissue will not absorb carbon-14 but will absorb carbon-12
E)All of the above are correct.
A)the amount of carbon-14 in all objects is the same
B)carbon-14 is very unstable and is readily lost from the atmosphere
C)the ratio of carbon-14 to carbon-12 in the atmosphere is a constant
D)living tissue will not absorb carbon-14 but will absorb carbon-12
E)All of the above are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which one of the following devices converts radioactive emissions to light for detection?
A)Geiger counter
B)photographic film
C)scintillation counter
D)radiotracer
E)none of the above
A)Geiger counter
B)photographic film
C)scintillation counter
D)radiotracer
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
51
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.What is the binding energy (in J)of a  Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
A)2.74 × 10-19
B)9.12 × 10-28
C)4.94 × 10-13
D)8.20 × 10-11
E)2.74 × 10-16
 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)A)2.74 × 10-19
B)9.12 × 10-28
C)4.94 × 10-13
D)8.20 × 10-11
E)2.74 × 10-16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
52
Cesium-137 undergoes beta decay and has a half-life of 30.0 years.How many beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes?
A)6.1 × 1013
B)6.2 × 1022
C)8.4 × 1015
D)1.3 × 10-8
E)8.1 × 1015
A)6.1 × 1013
B)6.2 × 1022
C)8.4 × 1015
D)1.3 × 10-8
E)8.1 × 1015

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
53
The half-life for beta decay of strontium-90 is 28.8 years.A milk sample is found to contain 10.3 ppm strontium-90.How many years would pass before the strontium-90 concentration would drop to 1.0 ppm?
A)92.3
B)0.112
C)186
D)96.9
E)131
A)92.3
B)0.112
C)186
D)96.9
E)131

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
54
The half-life of carbon-11 is 20.3 minutes.How much of a 100.0 mg sample remains after 1.50 hours?
A)8.48 mg
B)4.63 mg
C)12.9 mg
D)22.6 mg
E)7.70 mg
A)8.48 mg
B)4.63 mg
C)12.9 mg
D)22.6 mg
E)7.70 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
55
The half-life of a radionuclide ________.
A)is constant
B)gets shorter with passing time
C)gets longer with passing time
D)gets shorter with increased temperature
E)gets longer with increased temperature
A)is constant
B)gets shorter with passing time
C)gets longer with passing time
D)gets shorter with increased temperature
E)gets longer with increased temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
56
The half-life of 222Rn is 3.80 days.If a sample contains 36.0 g of Rn-222,how many years will it take for the sample to be reduced to 1.00 mg of Rn-222?
A)19.7
B)0.1575
C)8.53
D)0.0234
E)none of the above
A)19.7
B)0.1575
C)8.53
D)0.0234
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which one of the following is true?
A)Some spontaneous nuclear reactions are exothermic.
B)Some spontaneous nuclear reactions are endothermic.
C)All spontaneous nuclear reactions are exothermic.
D)There is no relationship between exothermicity and spontaneity in nuclear reactions.
E)All spontaneous nuclear reactions are endothermic.
A)Some spontaneous nuclear reactions are exothermic.
B)Some spontaneous nuclear reactions are endothermic.
C)All spontaneous nuclear reactions are exothermic.
D)There is no relationship between exothermicity and spontaneity in nuclear reactions.
E)All spontaneous nuclear reactions are endothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is a phosphor?
A)an oxide of phosphorus
B)a substance that thermally reduces to phosphorus
C)a bioluminescent substance
D)a substance that emits light when excited by radiation
E)an alkali metal phosphide
A)an oxide of phosphorus
B)a substance that thermally reduces to phosphorus
C)a bioluminescent substance
D)a substance that emits light when excited by radiation
E)an alkali metal phosphide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the rate constant (in min-1)for the decay of this radionuclide?
A)45
B)32
C)0.024
D)0.032
E)0.022
A)45
B)32
C)0.024
D)0.032
E)0.022

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
60
The half-life of 223Ra is 11.4 days.How much of a 200.0 mg sample remains after 600 hours?
A)0.219 mg
B)21.9 mg
C).0302 mg
D)43.8 mg
E)6.04 mg
A)0.219 mg
B)21.9 mg
C).0302 mg
D)43.8 mg
E)6.04 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
61
What is the identity of element E in the nuclear reaction 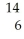 C → E +
C → E + 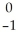 e?
e?
A)B
B)C
C)O
D)N
E)none of the above
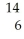 C → E +
C → E + 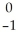 e?
e?A)B
B)C
C)O
D)N
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
62
The missing product from this reaction is ________.
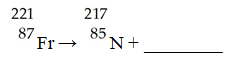
A)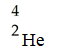
B)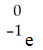
C)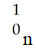
D)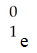
E)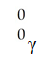
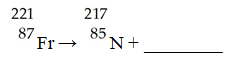
A)
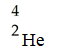
B)
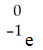
C)
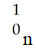
D)
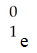
E)
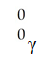

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
63
In terms of binding energy per nucleon,what element divides fission and fusion processes?
A)H
B)He
C)C
D)Fe
E)U
A)H
B)He
C)C
D)Fe
E)U

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
64
The missing reactant from this reaction is ________. 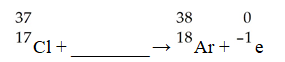
A)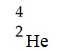
B)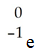
C)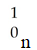
D)
E)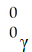

A)
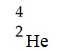
B)
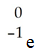
C)
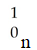
D)

E)
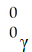

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
65
In balancing the nuclear reaction 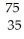 Br → E +
Br → E + 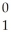 e,the identity of element E is ________.
e,the identity of element E is ________.
A)Kr
B)Br
C)U
D)Se
E)none of the above
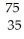 Br → E +
Br → E + 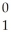 e,the identity of element E is ________.
e,the identity of element E is ________.A)Kr
B)Br
C)U
D)Se
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
66
The missing product from this reaction is ________.
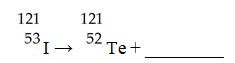
A)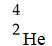
B)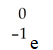
C)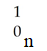
D)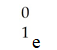
E)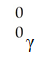
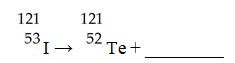
A)
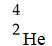
B)
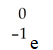
C)
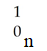
D)
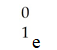
E)
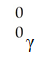

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
67
The missing product from this reaction is ________.
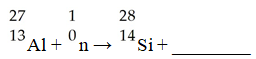
A)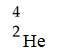
B)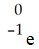
C)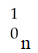
D)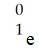
E)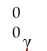
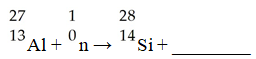
A)
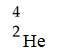
B)
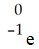
C)
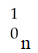
D)
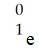
E)
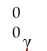

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which one of the following natural radionuclides is the most abundant?
A)Potassium-40
B)Rubidium-87
C)Thorium-232
D)Uranium-238
A)Potassium-40
B)Rubidium-87
C)Thorium-232
D)Uranium-238

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
69
By what process does thorium-230 decay to radium-226?
A)gamma emission
B)alpha emission
C)beta emission
D)electron capture
E)positron emission
A)gamma emission
B)alpha emission
C)beta emission
D)electron capture
E)positron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the mass defect (in amu)of a 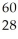 Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
A)0.5449
B)0.5505
C)1.2374
D)1.3066
E)28.7930
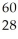 Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.A)0.5449
B)0.5505
C)1.2374
D)1.3066
E)28.7930

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
71
What percentage of electricity generated in the U.S.is from commercial nuclear plants?
A)1%
B)10%
C)19%
D)50%
E)90%
A)1%
B)10%
C)19%
D)50%
E)90%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
72
The missing reactant from this reaction is ________.
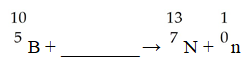
A)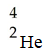
B)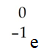
C)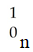
D)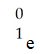
E)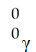
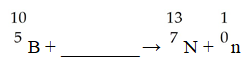
A)
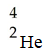
B)
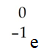
C)
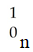
D)
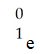
E)
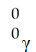

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
73
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.What is the binding energy per nucleon (in J)of a  Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
A)1.37 × 10-12
B)2.49 × 10-12
C)3.04 × 10-12
D)9.43 × 10-13
E)7.01 × 10-14
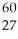 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)A)1.37 × 10-12
B)2.49 × 10-12
C)3.04 × 10-12
D)9.43 × 10-13
E)7.01 × 10-14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which one of the following forms of radiation can penetrate the deepest into body tissue?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
75
What type of reaction is known as a thermonuclear reaction?
A)fission
B)fusion
C)transmutation
D)beta emission
E)neutron emission
A)fission
B)fusion
C)transmutation
D)beta emission
E)neutron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
76
This reaction is an example of ________. 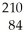 Po →
Po → 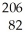 Pb + ________
Pb + ________
A)alpha decay
B)beta emission
C)gamma emission
D)positron emission
E)electron capture
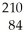 Po →
Po → 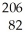 Pb + ________
Pb + ________A)alpha decay
B)beta emission
C)gamma emission
D)positron emission
E)electron capture

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
77
The main scientific difficulty in achieving a controlled fusion process is the ________.
A)enormous repulsion between nuclei being fused
B)enormous repulsion between the electrons of atoms being fused
C)very large number of positrons emitted
D)very large number of x-rays emitted
E)very large number of gamma rays emitted
A)enormous repulsion between nuclei being fused
B)enormous repulsion between the electrons of atoms being fused
C)very large number of positrons emitted
D)very large number of x-rays emitted
E)very large number of gamma rays emitted

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
78
In balancing the nuclear reaction 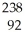 U →
U → 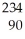 E +
E + 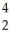 He,the identity of element E is ________.
He,the identity of element E is ________.
A)Pu
B)Np
C)U
D)Pa
E)Th
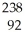 U →
U → 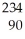 E +
E + 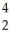 He,the identity of element E is ________.
He,the identity of element E is ________.A)Pu
B)Np
C)U
D)Pa
E)Th

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
79
What exposure level to radiation is fatal to most humans?
A)100 rem
B)200 rem
C)600 rem
D)300 rem
E)1000 rem
A)100 rem
B)200 rem
C)600 rem
D)300 rem
E)1000 rem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck
80
The alpha decay of what isotope of what element produces lead-206?
A)polonium-210
B)radon-222
C)mercury-202
D)bismuth-208
E)thallium-204
A)polonium-210
B)radon-222
C)mercury-202
D)bismuth-208
E)thallium-204

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 178 في هذه المجموعة.
فتح الحزمة
k this deck








