Deck 22: Chemistry of the Nonmetals
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/194
العب
ملء الشاشة (f)
Deck 22: Chemistry of the Nonmetals
1
Isotopes of hydrogen ________.
A)have the same atomic number and different mass numbers
B)have the same atomic number and the same mass number
C)have different atomic numbers and different mass numbers
D)have different atomic numbers and the same mass number
E)are exactly alike
A)have the same atomic number and different mass numbers
B)have the same atomic number and the same mass number
C)have different atomic numbers and different mass numbers
D)have different atomic numbers and the same mass number
E)are exactly alike
have the same atomic number and different mass numbers
2
Which of the following would produce an acidic solution?
A)Na2O and MgO
B)Na2O, MgO, and CaH2
C)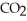 only
only
D)CaH2 only
E)CO, CO2, and CaH2
A)Na2O and MgO
B)Na2O, MgO, and CaH2
C)
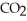 only
onlyD)CaH2 only
E)CO, CO2, and CaH2
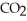 only
only 3
The heavier noble gases are more reactive than the lighter ones because ________.
A)the lighter noble gases exist as diatomic molecules
B)the lighter noble gases have complete octets
C)the heavier noble gases are more abundant
D)the heavier noble gases have low ionization energies relative to the lighter ones
E)the heavier noble gases have greater electron affinities
A)the lighter noble gases exist as diatomic molecules
B)the lighter noble gases have complete octets
C)the heavier noble gases are more abundant
D)the heavier noble gases have low ionization energies relative to the lighter ones
E)the heavier noble gases have greater electron affinities
the heavier noble gases have low ionization energies relative to the lighter ones
4
Of the atoms below,________ is the most effective in forming π bonds.
A)C
B)P
C)N
D)Si
E)Ge
A)C
B)P
C)N
D)Si
E)Ge

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which halogen is the most easily oxidized?
A)F
B)Cl
C)Br
D)I
A)F
B)Cl
C)Br
D)I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following compounds is the most stable?
A)XeOCl4
B)XeO2Cl2
C)XeO3
D)XeCl6
E)XeCl2
A)XeOCl4
B)XeO2Cl2
C)XeO3
D)XeCl6
E)XeCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
7
Consider the following xenon compounds:
(i)XeCl2
(ii)XeCl4
(iii)XeO4
(iv)XeOCl4
(v)XeO3
Which of the compounds is(are)polar?
A)(i)only
B)(iii)and (iv)
C)(iv)and (v)
D)(ii)and (iii)
E)(iv)only
(i)XeCl2
(ii)XeCl4
(iii)XeO4
(iv)XeOCl4
(v)XeO3
Which of the compounds is(are)polar?
A)(i)only
B)(iii)and (iv)
C)(iv)and (v)
D)(ii)and (iii)
E)(iv)only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
8
Chlorine can have a positive oxidation state ________.
A)if it combines with bromine or iodine
B)if it combines with oxygen or fluorine
C)if it combines with hydrogen
D)if it combines with an alkali metal
E)in its elemental form
A)if it combines with bromine or iodine
B)if it combines with oxygen or fluorine
C)if it combines with hydrogen
D)if it combines with an alkali metal
E)in its elemental form

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following would produce a basic solution?
A)Na2O and CaO
B)CO and CO2
C)CaH2 only
D)Na2O, CaO, and CaH2
E)CO, CO2, and CaH2
A)Na2O and CaO
B)CO and CO2
C)CaH2 only
D)Na2O, CaO, and CaH2
E)CO, CO2, and CaH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
10
The interhalogen compound ICl3 can form but BrCl3 cannot form.This is because ________.
A)iodine is large enough to accommodate three chlorine atoms around itself but bromine is not
B)bromine is not electronegative enough to react with chlorine
C)bromine is too electronegative to react with chlorine
D)iodine can have a positive oxidation state but bromine cannot
E)iodine can have a negative oxidation state but bromine cannot
A)iodine is large enough to accommodate three chlorine atoms around itself but bromine is not
B)bromine is not electronegative enough to react with chlorine
C)bromine is too electronegative to react with chlorine
D)iodine can have a positive oxidation state but bromine cannot
E)iodine can have a negative oxidation state but bromine cannot

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which noble gas is known to form a variety of binary compounds?
A)Xe
B)He
C)Ne
D)Ar
E)Kr
A)Xe
B)He
C)Ne
D)Ar
E)Kr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
12
Interhalogen compounds ________.
A)are exceedingly reactive
B)contain halogens in both positive and negative oxidation states
C)are powerful oxidizing agents
D)all of the above
A)are exceedingly reactive
B)contain halogens in both positive and negative oxidation states
C)are powerful oxidizing agents
D)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
13
Water gas is ________.
A)H2O and H2
B)CO2 and O2
C)H2O and CO2
D)H2O and CO
E)H2 and CO
A)H2O and H2
B)CO2 and O2
C)H2O and CO2
D)H2O and CO
E)H2 and CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
14
How are the oxygen-containing compounds of xenon made?
A)by direct combination of the elements
B)by reaction of xenon with peroxide
C)by thermal decomposition of the xenon hydroxide
D)by reaction of the corresponding xenon fluoride with water
E)Xenon is inert and does not form compounds with oxygen.
A)by direct combination of the elements
B)by reaction of xenon with peroxide
C)by thermal decomposition of the xenon hydroxide
D)by reaction of the corresponding xenon fluoride with water
E)Xenon is inert and does not form compounds with oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which elemental halogen(s)can be used to prepare 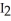 from NaI?
from NaI?
A)F2 only
B)Cl2 only
C)Br2 only
D)both Cl2 and Br2, but not F2
E)F2, Cl2, and Br2
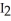 from NaI?
from NaI?A)F2 only
B)Cl2 only
C)Br2 only
D)both Cl2 and Br2, but not F2
E)F2, Cl2, and Br2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which equation correctly represents the reaction between silica and hydrofluoric acid?
A)SiCl4 + 4HF → SiF4 + 4HCl
B)SiO2 + 6HF → H2SiF6 + 2H2O
C)SiCl2 + 2HF → SiF2 + 2HCl
D)SiH4 + 4HF → SiF4 + 4H2
E)none of the above
A)SiCl4 + 4HF → SiF4 + 4HCl
B)SiO2 + 6HF → H2SiF6 + 2H2O
C)SiCl2 + 2HF → SiF2 + 2HCl
D)SiH4 + 4HF → SiF4 + 4H2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
17
How many oxygen atoms are bonded to each silicon atom in SiO2?
A)1
B)2
C)3
D)4
E)none
A)1
B)2
C)3
D)4
E)none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
18
What method is used to produce the most hydrogen gas in the United States?
A)electrolysis of water
B)reaction of zinc with acid
C)reaction of methane with steam
D)reaction of coke (carbon)with steam
E)reaction of metallic sodium with water
A)electrolysis of water
B)reaction of zinc with acid
C)reaction of methane with steam
D)reaction of coke (carbon)with steam
E)reaction of metallic sodium with water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the primary commercial use of hydrogen in the United States?
A)as a rocket fuel, especially on the space shuttle
B)hydrogenation of vegetable oils
C)manufacture of methanol
D)manufacture of ammonia by the Haber process
E)as an automobile fuel
A)as a rocket fuel, especially on the space shuttle
B)hydrogenation of vegetable oils
C)manufacture of methanol
D)manufacture of ammonia by the Haber process
E)as an automobile fuel

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which one of the following is false concerning tritium?
A)It is radioactive, emitting alpha particles with a half-life of 12.3 yr.
B)It can be produced by neutron bombardment of lithium-6.
C)It has the same chemical properties as protium but reacts more slowly.
D)The atomic number of tritium is 1.
A)It is radioactive, emitting alpha particles with a half-life of 12.3 yr.
B)It can be produced by neutron bombardment of lithium-6.
C)It has the same chemical properties as protium but reacts more slowly.
D)The atomic number of tritium is 1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the major commercial source of elemental sulfur?
A)sulfide minerals
B)sulfate minerals
C)underground deposits of elemental sulfur
D)seawater
E)coal and petroleum
A)sulfide minerals
B)sulfate minerals
C)underground deposits of elemental sulfur
D)seawater
E)coal and petroleum

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
22
________ are typically basic while ________ are typically acidic.
A)Nonmetal oxides, metal oxides
B)Metals, nonmetals
C)Metal oxides, nonmetal oxides
D)Nonmetals, metals
A)Nonmetal oxides, metal oxides
B)Metals, nonmetals
C)Metal oxides, nonmetal oxides
D)Nonmetals, metals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which one of the following is true concerning pure hydrazine?
A)It is a weak reducing agent.
B)It can be made by reaction of nitrogen and ammonia.
C)It is used as a rocket fuel.
D)It is a hydride of oxygen.
E)It is a hydride of ammonia.
A)It is a weak reducing agent.
B)It can be made by reaction of nitrogen and ammonia.
C)It is used as a rocket fuel.
D)It is a hydride of oxygen.
E)It is a hydride of ammonia.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
24
The oxidation number of N in NO3- is ________.
A)+1
B)+2
C)+3
D)+4
E)+5
A)+1
B)+2
C)+3
D)+4
E)+5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
25
The Ostwald process is by which NH3 is converted commercially into ________.
A)HNO3
B)NO
C)NO2
D)N2O3
E)N2O5
A)HNO3
B)NO
C)NO2
D)N2O3
E)N2O5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
26
The oxidation state of fluorine in its compounds is ________.
A)positive unless it combines with another halogen
B)negative unless it combines with another halogen
C)negative unless it combines with oxygen
D)negative unless it combines with an active metal
E)always negative
A)positive unless it combines with another halogen
B)negative unless it combines with another halogen
C)negative unless it combines with oxygen
D)negative unless it combines with an active metal
E)always negative

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which form of elemental sulfur is the most stable at room temperature?
A)rhombic sulfur
B)monoclinic
C)hexagonal
D)triclinic
E)tetraclinic
A)rhombic sulfur
B)monoclinic
C)hexagonal
D)triclinic
E)tetraclinic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
28
The prefix thio- denotes ________.
A)replacement of an oxygen atom by a sulfur atom
B)a sulfur-sulfur double bond
C)sulfur in a negative oxidation state
D)a sulfur-oxygen double bond
E)an allotropic form of sulfur
A)replacement of an oxygen atom by a sulfur atom
B)a sulfur-sulfur double bond
C)sulfur in a negative oxidation state
D)a sulfur-oxygen double bond
E)an allotropic form of sulfur

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following statements is false?
A)Ozone is a better reducing agent than O2 (g).
B)Ozone is produced by passing electricity through dry O2 (g).
C)Ozone oxidizes all of the common metals except gold and platinum.
D)Ozone decomposes to O2 and O.
E)Ozone is an allotrope of oxygen.
A)Ozone is a better reducing agent than O2 (g).
B)Ozone is produced by passing electricity through dry O2 (g).
C)Ozone oxidizes all of the common metals except gold and platinum.
D)Ozone decomposes to O2 and O.
E)Ozone is an allotrope of oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following react with oxygen to form superoxides?
A)Ca
B)Na
C)K
D)Sr
E)Ba
A)Ca
B)Na
C)K
D)Sr
E)Ba

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
31
Amphoteric oxides are also known as ________.
A)basic oxides
B)basic anhydrides
C)acidic oxides
D)acidic anhydrides
E)none of the above
A)basic oxides
B)basic anhydrides
C)acidic oxides
D)acidic anhydrides
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
32
Nearly all commercial oxygen is obtained ________.
A)from air
B)by electrolysis of water
C)by thermal decomposition of potassium chlorate
D)by thermal cracking of petroleum
E)as a byproduct of the preparation of aluminum in the Hall process
A)from air
B)by electrolysis of water
C)by thermal decomposition of potassium chlorate
D)by thermal cracking of petroleum
E)as a byproduct of the preparation of aluminum in the Hall process

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
33
The most stable allotrope of oxygen is ________.
A)H2O
B)O3
C)O2
D)HClO
E)O
A)H2O
B)O3
C)O2
D)HClO
E)O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
34
The oxidation numbers of sulfur in the sulfate ion,sulfite ion,sulfur trioxide,and hydrogen sulfide are ________,________,________,and ________,respectively.
A)+4, -2, +4, +6
B)+6, +2, +4, +6
C)+6, +4, +6, -2
D)+4, +6, +4, -2
E)-2, +6, -2, 0
A)+4, -2, +4, +6
B)+6, +2, +4, +6
C)+6, +4, +6, -2
D)+4, +6, +4, -2
E)-2, +6, -2, 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which one of the following is sodium thiosulfate?
A)Na2SO4
B)Na2SO3
C)Na2S2O3
D)Na2S4O6
E)Na2S
A)Na2SO4
B)Na2SO3
C)Na2S2O3
D)Na2S4O6
E)Na2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
36
What sulfur compound is used to sterilize wine?
A)H2SO4
B)H2S
C)Na2SO3
D)SO2
E)Na2S
A)H2SO4
B)H2S
C)Na2SO3
D)SO2
E)Na2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which element in group 6A is not capable of having an expanded valence shell?
A)sulfur
B)tellurium
C)selenium
D)oxygen
E)polonium
A)sulfur
B)tellurium
C)selenium
D)oxygen
E)polonium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
38
A disproportionation reaction is one in which ________.
A)a single element is both oxidized and reduced
B)a compound is separated into its constituent elements
C)the ratio of combination of two elements in a compound changes
D)aqueous ions combine to form an insoluble salt
E)an insoluble salt separates into ions
A)a single element is both oxidized and reduced
B)a compound is separated into its constituent elements
C)the ratio of combination of two elements in a compound changes
D)aqueous ions combine to form an insoluble salt
E)an insoluble salt separates into ions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which element in group 7A is not capable of having an expanded valence shell?
A)astatine
B)fluorine
C)chlorine
D)bromine
E)iodine
A)astatine
B)fluorine
C)chlorine
D)bromine
E)iodine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
40
The oxidation state of oxygen in OF2 is ________.
A)+1
B)+2
C)0
D)-1
E)-2
A)+1
B)+2
C)0
D)-1
E)-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which one of the following is false concerning buckminsterfullerene?
A)It is the most recently discovered crystalline allotrope of carbon.
B)It consists of individual molecules like C60 and C70.
C)It is a molecular form of carbon.
D)It is made up of Cl2 molecules.
E)It is made up of molecules that resemble soccer balls.
A)It is the most recently discovered crystalline allotrope of carbon.
B)It consists of individual molecules like C60 and C70.
C)It is a molecular form of carbon.
D)It is made up of Cl2 molecules.
E)It is made up of molecules that resemble soccer balls.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
42
What are the products of the reaction of PF3 (g)and water?
A)phosphorous acid and hydrofluoric acid
B)elemental phosphorus and hydrofluoric acid
C)phosphoric acid and fluorine gas
D)elemental phosphorus and fluorine gas
E)phosphoric acid and phosphorous acid
A)phosphorous acid and hydrofluoric acid
B)elemental phosphorus and hydrofluoric acid
C)phosphoric acid and fluorine gas
D)elemental phosphorus and fluorine gas
E)phosphoric acid and phosphorous acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
43
Carbon dioxide is produced ________.
A)in blast furnaces when metal oxides are reduced with CO
B)by combustion of carbon-containing substances in an excess of oxygen
C)when carbonates are heated
D)by fermentation of sugar during the production of ethanol
E)by all of the above processes
A)in blast furnaces when metal oxides are reduced with CO
B)by combustion of carbon-containing substances in an excess of oxygen
C)when carbonates are heated
D)by fermentation of sugar during the production of ethanol
E)by all of the above processes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following is not an allotropic form of carbon?
A)graphite
B)carbon dioxide
C)diamond
D)buckminsterfullerene
E)All of the above are allotropic forms of carbon.
A)graphite
B)carbon dioxide
C)diamond
D)buckminsterfullerene
E)All of the above are allotropic forms of carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
45
Of the following substances,________ is both a very strong acid and a powerful oxidizing agent.
A)HNO3
B)H2SO4
C)HCl
D)H3PO4
E)HF
A)HNO3
B)H2SO4
C)HCl
D)H3PO4
E)HF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
46
The oxidation numbers of nitrogen in the nitride ion,hydrazine,ammonium cation,and nitrate ion are ________,________,________,and ________,respectively.
A)-3, -2, -3, +5
B)+3, -2, -3, +5
C)+3, -2, +1, +3
D)-3, +2, +1, +5
E)-3, +2, -3, +3
A)-3, -2, -3, +5
B)+3, -2, -3, +5
C)+3, -2, +1, +3
D)-3, +2, +1, +5
E)-3, +2, -3, +3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following equations correctly represents the combustion of hydrazine?
A)N2H4 (l)+ O2 (g)→ NH3 (g)+ HNO2 (g)
B)N2H4 (l)+ 2O2 (g)→ 2NO2 (g)+ 2H2 (g)
C)N2H4 (l)+ O2 (g)→ 2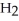 NO (g)
NO (g)
D)N2H4 (l)+ O2 (g)→ N2 (g)+ 2 H2O (g)
E)N2H4 (l)+ O2 (g)→ N2 (g)+ 2H2 (g)+ O2 (g)
A)N2H4 (l)+ O2 (g)→ NH3 (g)+ HNO2 (g)
B)N2H4 (l)+ 2O2 (g)→ 2NO2 (g)+ 2H2 (g)
C)N2H4 (l)+ O2 (g)→ 2
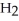 NO (g)
NO (g)D)N2H4 (l)+ O2 (g)→ N2 (g)+ 2 H2O (g)
E)N2H4 (l)+ O2 (g)→ N2 (g)+ 2H2 (g)+ O2 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following statements best describes graphite?
A)It is a soft, slippery, and black solid that has a metallic luster and conducts electricity.
B)It is a soft, slippery, and black gas that has a metallic luster and conducts electricity.
C)It is a soft, slippery, and black liquid that has a metallic luster and conducts electricity.
D)It is a soft, slippery, and black solid that has a metallic luster and does not conduct electricity.
E)It is a soft, slippery, and black solid that has a nonmetallic dull look and does not conduct electricity.
A)It is a soft, slippery, and black solid that has a metallic luster and conducts electricity.
B)It is a soft, slippery, and black gas that has a metallic luster and conducts electricity.
C)It is a soft, slippery, and black liquid that has a metallic luster and conducts electricity.
D)It is a soft, slippery, and black solid that has a metallic luster and does not conduct electricity.
E)It is a soft, slippery, and black solid that has a nonmetallic dull look and does not conduct electricity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following would produce the most strongly acidic aqueous solution?
A)HCO3-
B)CO
C)CO2
D)CO32-
E)CaCO3
A)HCO3-
B)CO
C)CO2
D)CO32-
E)CaCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
50
The reaction between nitrogen dioxide and water is a ________.
A)decomposition
B)combustion
C)disproportionation
D)neutralization
E)replacement
A)decomposition
B)combustion
C)disproportionation
D)neutralization
E)replacement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
51
Of the following,which is most likely to form interstitial carbides?
A)active metals
B)transition metals
C)boron and silicon
D)alkaline earth metals
E)alkali metals
A)active metals
B)transition metals
C)boron and silicon
D)alkaline earth metals
E)alkali metals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
52
How many lone pair of electrons are there in one molecule of carbon monoxide?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
53
The oxidation number of N in NO2- is ________.
A)+1
B)+2
C)+3
D)+4
E)+5
A)+1
B)+2
C)+3
D)+4
E)+5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of the following would produce the strongest basic aqueous solution?
A)CO
B)CO2
C)H2CO3
D)Na2CO3
E)KHCO3
A)CO
B)CO2
C)H2CO3
D)Na2CO3
E)KHCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which equation correctly represents the reaction between carbon dioxide and water?
A)CO2 (aq)+ H2O (l)→ H2CO3 (aq)
B)CO2 (aq)+ H2O (l)→ H2 (g)+ CO (g)+ O2 (g)
C)CO2 (aq)+ H2O (l)→ H2O2 (aq)+ CO (g)
D)CO2 (aq)+ 2H2O (l)→ CH4 (g)+ 2O2 (aq)
E)CO2 (aq)+ H2O (l)→ H2CO (aq)+ O2 (g)
A)CO2 (aq)+ H2O (l)→ H2CO3 (aq)
B)CO2 (aq)+ H2O (l)→ H2 (g)+ CO (g)+ O2 (g)
C)CO2 (aq)+ H2O (l)→ H2O2 (aq)+ CO (g)
D)CO2 (aq)+ 2H2O (l)→ CH4 (g)+ 2O2 (aq)
E)CO2 (aq)+ H2O (l)→ H2CO (aq)+ O2 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which equation correctly represents what happens when N 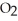 dissolves in water?
dissolves in water?
A)NO2 (g)+ H2O (l)→ 2H+ (aq)+ NO3- (aq)
B)3NO2 (g)+ H2O (l)→ 2H+ (aq)+ 2NO3- (aq)+ NO (g)
C)NO2 (g)+ H2O (l)→ H2O2 (aq)+ NO (g)
D)2NO2 (g)+ H2O (l)→ 2H+ (aq)+ NO42- (aq)+ NO (g)
E)2NO2 (g)+ 2H2O (l)→ 2HNO2 (aq)+ O2 (g)+ H2 (g)
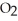 dissolves in water?
dissolves in water?A)NO2 (g)+ H2O (l)→ 2H+ (aq)+ NO3- (aq)
B)3NO2 (g)+ H2O (l)→ 2H+ (aq)+ 2NO3- (aq)+ NO (g)
C)NO2 (g)+ H2O (l)→ H2O2 (aq)+ NO (g)
D)2NO2 (g)+ H2O (l)→ 2H+ (aq)+ NO42- (aq)+ NO (g)
E)2NO2 (g)+ 2H2O (l)→ 2HNO2 (aq)+ O2 (g)+ H2 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which phosphorus chloride compound will form in the reaction of phosphorus with excess chlorine gas?
A)PCl3
B)PCl2
C)PCl5
D)PCl4
E)PCl
A)PCl3
B)PCl2
C)PCl5
D)PCl4
E)PCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
58
What are the products of the reaction of PCl5 (g)and water?
A)phosphoric acid and phosphorous acid
B)elemental phosphorus and hydrochloric acid
C)phosphoric acid and chlorine gas
D)elemental phosphorus and chlorine gas
E)phosphoric acid and hydrochloric acid
A)phosphoric acid and phosphorous acid
B)elemental phosphorus and hydrochloric acid
C)phosphoric acid and chlorine gas
D)elemental phosphorus and chlorine gas
E)phosphoric acid and hydrochloric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
59
The oxidation number of Pb in PbO2 is ________.
A)+1
B)+2
C)+3
D)+4
E)+5
A)+1
B)+2
C)+3
D)+4
E)+5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the function of the carbon fibers in a composite?
A)to provide a structure to help the epoxy resin solidify in the desired shape
B)to transmit loads evenly in all directions
C)to provide resistance to oxidation
D)to provide ultraviolet protection
E)to "spread out" the epoxy so that it remains more flexible
A)to provide a structure to help the epoxy resin solidify in the desired shape
B)to transmit loads evenly in all directions
C)to provide resistance to oxidation
D)to provide ultraviolet protection
E)to "spread out" the epoxy so that it remains more flexible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
61
Hydrogen can form hydride ions.Elements in group ________ typically form ions with the same charge as the hydride ion.
A)1A
B)2A
C)6A
D)7A
E)3A
A)1A
B)2A
C)6A
D)7A
E)3A

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
62
In metallic hydrides,the oxidation number of hydrogen is considered to be ________.
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
63
B2O3 is the anhydride of ________.
A)borous acid
B)diborane
C)tetraboric acid
D)boric acid
E)borax
A)borous acid
B)diborane
C)tetraboric acid
D)boric acid
E)borax

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
64
Sodium borohydride,NaB 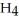 ,is a strong reducing agent because ________.
,is a strong reducing agent because ________.
A)Na+ is easily reduced to Na (s)
B)boron easily changes its oxidation number from +3 to -3
C)boron is readily oxidized from -3 oxidation state to +3
D)hydrogen can be easily oxidized from -1 oxidation state to +1
E)hydrogen is easily reduced from +1 oxidation state to 0
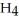 ,is a strong reducing agent because ________.
,is a strong reducing agent because ________.A)Na+ is easily reduced to Na (s)
B)boron easily changes its oxidation number from +3 to -3
C)boron is readily oxidized from -3 oxidation state to +3
D)hydrogen can be easily oxidized from -1 oxidation state to +1
E)hydrogen is easily reduced from +1 oxidation state to 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
65
Boric acid condenses to form tetraboric acid according to the equation ________.
A)4H3BO3 (s)→ 2H2B2O7 (s)+ 3H2O (g)
B)2H3BO3 (s)→ HB2O2 (s)+ 4H2O (g)
C)4H3BO3 (s)→ HB4O8 (s)+ 4H2O (g)
D)2H3BO3 (s)→ H2B4O7 (s)+ 3H2O (g)
E)4H3BO3 (s)→ H2B4O7 (s)+ 5H2O (g)
A)4H3BO3 (s)→ 2H2B2O7 (s)+ 3H2O (g)
B)2H3BO3 (s)→ HB2O2 (s)+ 4H2O (g)
C)4H3BO3 (s)→ HB4O8 (s)+ 4H2O (g)
D)2H3BO3 (s)→ H2B4O7 (s)+ 3H2O (g)
E)4H3BO3 (s)→ H2B4O7 (s)+ 5H2O (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
66
Addition of 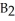
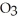 to soda-lime glass ________.
to soda-lime glass ________.
A)imparts a greater ability to withstand temperature change
B)imparts a deep blue color
C)results in a denser glass with a higher refractive index
D)results in a glass with a lower melting point
E)results in opaque glass
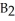
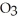 to soda-lime glass ________.
to soda-lime glass ________.A)imparts a greater ability to withstand temperature change
B)imparts a deep blue color
C)results in a denser glass with a higher refractive index
D)results in a glass with a lower melting point
E)results in opaque glass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
67
The primary commercial use of nitric acid is ________.
A)in the manufacture of plastics
B)in the manufacture of explosives
C)in pool water maintenance
D)in the manufacture of fertilizers
E)in the manufacture of anti-depressant drugs
A)in the manufacture of plastics
B)in the manufacture of explosives
C)in pool water maintenance
D)in the manufacture of fertilizers
E)in the manufacture of anti-depressant drugs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
68
Soda-lime glass contains ________.
A)SiO2 and aluminum
B)SiO2, CaO, and Na2O
C)SiO2, CO2, and citric acid
D)SiO2, CO2, Na2O
E)pure SiO2
A)SiO2 and aluminum
B)SiO2, CaO, and Na2O
C)SiO2, CO2, and citric acid
D)SiO2, CO2, Na2O
E)pure SiO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
69
Silicones can be oils or rubber-like materials depending on ________.
A)the silicon-to-oxygen ratio
B)the length of the chain and degree of cross-linking
C)the percentage of carbon in the chain
D)the percentage of sulfur in the chain
E)the oxidation state of silicon in the chain
A)the silicon-to-oxygen ratio
B)the length of the chain and degree of cross-linking
C)the percentage of carbon in the chain
D)the percentage of sulfur in the chain
E)the oxidation state of silicon in the chain

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
70
A borane is a ________.
A)compound containing only boron and oxygen
B)compound containing only boron and aluminum
C)compound containing only boron and hydrogen
D)compound containing only boron and carbon
E)three-dimensional covalent network of boron atoms
A)compound containing only boron and oxygen
B)compound containing only boron and aluminum
C)compound containing only boron and hydrogen
D)compound containing only boron and carbon
E)three-dimensional covalent network of boron atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
71
The most common isotope of hydrogen is sometimes referred to as ________.
A)deuterium
B)protium
C)tritium
D)heavy hydrogen
E)common hydrogen
A)deuterium
B)protium
C)tritium
D)heavy hydrogen
E)common hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
72
Hydrogen can have oxidation states of ________.
A)+1 only
B)-1, 0, and +1
C)0 and +1 only
D)-1 and +1 only
E)0 only
A)+1 only
B)-1, 0, and +1
C)0 and +1 only
D)-1 and +1 only
E)0 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
73
Additives can be used in soda-lime glass to alter its ________.
A)ability to withstand temperature change
B)color
C)hardness
D)melting point
E)any of the above
A)ability to withstand temperature change
B)color
C)hardness
D)melting point
E)any of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following equations correctly represents the reaction of 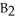
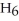 with oxygen?
with oxygen?
A)B2H6 (g)+ 3O2 (g)→ B2O3 (s)+ 3H2O (g)
B)B2H6 (g)+ O2 (g)→ B2O2 (s)+ 3H2 (g)
C)B2H6 (g)+ 2O2 (g)→ B2H2 (s)+ 2H2O2 (aq)
D)B2H6 (g)+ 2O2 (g)→ B2O2 (s)+ 3H2 + O2 (g)
E)B2H6 (g)+ O2 (g)→ H2B2O2 (s)+ 2H2 (g)
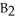
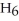 with oxygen?
with oxygen?A)B2H6 (g)+ 3O2 (g)→ B2O3 (s)+ 3H2O (g)
B)B2H6 (g)+ O2 (g)→ B2O2 (s)+ 3H2 (g)
C)B2H6 (g)+ 2O2 (g)→ B2H2 (s)+ 2H2O2 (aq)
D)B2H6 (g)+ 2O2 (g)→ B2O2 (s)+ 3H2 + O2 (g)
E)B2H6 (g)+ O2 (g)→ H2B2O2 (s)+ 2H2 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
75
Hydrogen can combine with ________ to form a metallic hydride.
A)an element from group 5A
B)an element from group 7A
C)an element from group 8A
D)an element from group 1A
E)an element from group 6A
A)an element from group 5A
B)an element from group 7A
C)an element from group 8A
D)an element from group 1A
E)an element from group 6A

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
76
Silicones are ________.
A)chains of alternating silicon and oxygen atoms with attached organic groups
B)three-dimensional covalent networks of SiO4 tetrahedra
C)three-dimensional covalent networks of silicon atoms
D)flat sheets of silicon atoms
E)flat sheets of silicon and hydrogen atoms
A)chains of alternating silicon and oxygen atoms with attached organic groups
B)three-dimensional covalent networks of SiO4 tetrahedra
C)three-dimensional covalent networks of silicon atoms
D)flat sheets of silicon atoms
E)flat sheets of silicon and hydrogen atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which one of the following is true concerning borax?
A)It is the hydrated sodium salt of tetraboric acid.
B)It is found in dry lake deposits in California.
C)Its aqueous solutions are alkaline.
D)It is commonly used in cleaning products.
E)All of the above are true.
A)It is the hydrated sodium salt of tetraboric acid.
B)It is found in dry lake deposits in California.
C)Its aqueous solutions are alkaline.
D)It is commonly used in cleaning products.
E)All of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
78
The arrangement of oxygen atoms around a silicon atom in SiO44- is ________.
A)square planar
B)octahedral
C)linear
D)tetrahedral
E)trigonal pyramidal
A)square planar
B)octahedral
C)linear
D)tetrahedral
E)trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
79
Replacement of CaO by PbO in soda-lime glass results in ________.
A)denser glass with a higher refractive index
B)glass with a deep blue color
C)opaque glass
D)a softer glass with a lower melting point
E)a harder glass with a higher melting point
A)denser glass with a higher refractive index
B)glass with a deep blue color
C)opaque glass
D)a softer glass with a lower melting point
E)a harder glass with a higher melting point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck
80
________ can violate the octet rule in its compounds.
A)carbon
B)nitrogen
C)boron
D)oxygen
E)none of these elements can violate the octet rule.
A)carbon
B)nitrogen
C)boron
D)oxygen
E)none of these elements can violate the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 194 في هذه المجموعة.
فتح الحزمة
k this deck








