Deck 2: Atoms, molecules, and Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/249
العب
ملء الشاشة (f)
Deck 2: Atoms, molecules, and Ions
1
The charge on an electron was determined in the ________.
A)cathode ray tube, by J. J. Thomson
B)Rutherford gold foil experiment
C)Millikan oil drop experiment
D)Dalton atomic theory
E)atomic theory of matter
A)cathode ray tube, by J. J. Thomson
B)Rutherford gold foil experiment
C)Millikan oil drop experiment
D)Dalton atomic theory
E)atomic theory of matter
Millikan oil drop experiment
2
Which one of the following is not one of the postulates of Dalton's atomic theory?
A)Atoms are composed of protons, neutrons, and electrons.
B)All atoms of a given element are identical; the atoms of different elements are different and have different properties.
C)Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions.
D)Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.
E)Each element is composed of extremely small particles called atoms.
A)Atoms are composed of protons, neutrons, and electrons.
B)All atoms of a given element are identical; the atoms of different elements are different and have different properties.
C)Atoms of an element are not changed into different types of atoms by chemical reactions: atoms are neither created nor destroyed in chemical reactions.
D)Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.
E)Each element is composed of extremely small particles called atoms.
Atoms are composed of protons, neutrons, and electrons.
3
Which one of the following is not true concerning cathode rays?
A)They originate from the negative electrode.
B)They travel in straight lines in the absence of electric or magnetic fields.
C)They impart a negative charge to metals exposed to them.
D)They are made up of electrons.
E)The characteristics of cathode rays depend on the material from which they are emitted.
A)They originate from the negative electrode.
B)They travel in straight lines in the absence of electric or magnetic fields.
C)They impart a negative charge to metals exposed to them.
D)They are made up of electrons.
E)The characteristics of cathode rays depend on the material from which they are emitted.
The characteristics of cathode rays depend on the material from which they are emitted.
4
Of the three types of radioactivity characterized by Rutherford,which type does not become deflected by a electric field?
A)β-rays
B)α-rays and β-rays
C)α-rays
D)γ-rays
E)α-rays, β-rays, and γ-rays
A)β-rays
B)α-rays and β-rays
C)α-rays
D)γ-rays
E)α-rays, β-rays, and γ-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
5
Of the following,the smallest and lightest subatomic particle is the ________.
A)neutron
B)proton
C)electron
D)nucleus
E)alpha particle
A)neutron
B)proton
C)electron
D)nucleus
E)alpha particle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
6
Cathode rays are deflected away from a negatively charged plate because ________.
A)they are not particles
B)they are positively charged particles
C)they are neutral particles
D)they are negatively charged particles
E)they are emitted by all matter
A)they are not particles
B)they are positively charged particles
C)they are neutral particles
D)they are negatively charged particles
E)they are emitted by all matter

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
7
In the absence of magnetic or electric fields,cathode rays ________.
A)do not exist
B)travel in straight lines
C)cannot be detected
D)become positively charged
E)bend toward a light source
A)do not exist
B)travel in straight lines
C)cannot be detected
D)become positively charged
E)bend toward a light source

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
8
The gold foil experiment performed in Rutherford's lab ________.
A)confirmed the plum-pudding model of the atom
B)led to the discovery of the atomic nucleus
C)was the basis for Thomson's model of the atom
D)utilized the deflection of beta particles by gold foil
E)proved the law of multiple proportions
A)confirmed the plum-pudding model of the atom
B)led to the discovery of the atomic nucleus
C)was the basis for Thomson's model of the atom
D)utilized the deflection of beta particles by gold foil
E)proved the law of multiple proportions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which statement below correctly describes the responses of alpha,beta,and gamma radiation to an electric field?
A)Both beta and gamma are deflected in the same direction, while alpha shows no response.
B)Both alpha and gamma are deflected in the same direction, while beta shows no response.
C)Both alpha and beta are deflected in the same direction, while gamma shows no response.
D)Alpha and beta are deflected in opposite directions, while gamma shows no response.
E)Only alpha is deflected, while beta and gamma show no response.
A)Both beta and gamma are deflected in the same direction, while alpha shows no response.
B)Both alpha and gamma are deflected in the same direction, while beta shows no response.
C)Both alpha and beta are deflected in the same direction, while gamma shows no response.
D)Alpha and beta are deflected in opposite directions, while gamma shows no response.
E)Only alpha is deflected, while beta and gamma show no response.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
10
________-rays consist of fast-moving electrons.
A)Alpha
B)Beta
C)Gamma
D)X
E)none of the above
A)Alpha
B)Beta
C)Gamma
D)X
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
11
In the Rutherford nuclear-atom model,________.
A)the heavy subatomic particles, protons and neutrons, reside in the nucleus
B)the three principal subatomic particles (protons, neutrons, and electrons)all have essentially the same mass
C)the light subatomic particles, protons and neutrons, reside in the nucleus
D)mass is spread essentially uniformly throughout the atom
E)the three principal subatomic particles (protons, neutrons, and electrons)all have essentially the same mass and mass is spread essentially uniformly throughout the atom
A)the heavy subatomic particles, protons and neutrons, reside in the nucleus
B)the three principal subatomic particles (protons, neutrons, and electrons)all have essentially the same mass
C)the light subatomic particles, protons and neutrons, reside in the nucleus
D)mass is spread essentially uniformly throughout the atom
E)the three principal subatomic particles (protons, neutrons, and electrons)all have essentially the same mass and mass is spread essentially uniformly throughout the atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
12
Consider the following selected postulates of Dalton's atomic theory:
(i)Each element is composed of extremely small particles called atoms.
(ii)Atoms are indivisible.
(iii)Atoms of a given element are identical.
(iv)Atoms of different elements are different and have different properties.
Which of the postulates is(are)no longer considered valid?
A)(i)and (ii)
B)(ii)only
C)(ii)and (iii)
D)(iii)only
E)(iii)and (iv)
(i)Each element is composed of extremely small particles called atoms.
(ii)Atoms are indivisible.
(iii)Atoms of a given element are identical.
(iv)Atoms of different elements are different and have different properties.
Which of the postulates is(are)no longer considered valid?
A)(i)and (ii)
B)(ii)only
C)(ii)and (iii)
D)(iii)only
E)(iii)and (iv)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
13
Cathode rays are ________.
A)neutrons
B)X-rays
C)electrons
D)protons
E)atoms
A)neutrons
B)X-rays
C)electrons
D)protons
E)atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which atom has the smallest number of neutrons?
A)carbon-14
B)nitrogen-14
C)oxygen-16
D)fluorine-19
E)neon-20
A)carbon-14
B)nitrogen-14
C)oxygen-16
D)fluorine-19
E)neon-20

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
15
All atoms of a given element have the same ________.
A)mass
B)number of protons
C)number of neutrons
D)number of electrons and neutrons
E)density
A)mass
B)number of protons
C)number of neutrons
D)number of electrons and neutrons
E)density

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which pair of substances could be used to illustrate the law of multiple proportions?
A)SO2, H2SO4
B)CO, CO2
C)H2O, O2
D)CH4, C6H12O6
E)NaCl, KCl
A)SO2, H2SO4
B)CO, CO2
C)H2O, O2
D)CH4, C6H12O6
E)NaCl, KCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
17
Of the three types of radioactivity characterized by Rutherford,which is/are not electrically charged?
A)α-rays
B)α-rays, β-rays, and γ-rays
C)γ-rays
D)α-rays and β-rays
E)α-rays and γ-rays
A)α-rays
B)α-rays, β-rays, and γ-rays
C)γ-rays
D)α-rays and β-rays
E)α-rays and γ-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
18
Of the three types of radioactivity characterized by Rutherford,which is/are electrically charged?
A)β-rays
B)α-rays and β-rays
C)α-rays, β-rays, and γ-rays
D)α-rays
E)α-rays and γ-rays
A)β-rays
B)α-rays and β-rays
C)α-rays, β-rays, and γ-rays
D)α-rays
E)α-rays and γ-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
19
A molecule of water contains hydrogen and oxygen in a 1:8 ratio by mass.This is a statement of ________.
A)the law of multiple proportions
B)the law of constant composition
C)the law of conservation of mass
D)the law of conservation of energy
E)none of the above
A)the law of multiple proportions
B)the law of constant composition
C)the law of conservation of mass
D)the law of conservation of energy
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
20
Of the three types of radioactivity characterized by Rutherford,which are particles?
A)β-rays
B)α-rays, β-rays, and γ-rays
C)γ-rays
D)α-rays and γ-rays
E)α-rays and β-rays
A)β-rays
B)α-rays, β-rays, and γ-rays
C)γ-rays
D)α-rays and γ-rays
E)α-rays and β-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which pair of atoms constitutes a pair of isotopes of the same element?
A)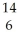 X
X 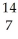 X
X
B)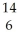 X
X 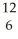 X
X
C)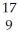 X
X 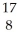 X
X
D) X
X  X
X
E)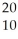 X
X 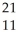 X
X
A)
 X
X  X
XB)
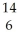 X
X 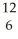 X
XC)
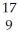 X
X 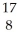 X
XD)
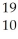 X
X 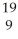 X
XE)
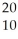 X
X 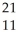 X
X
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
22
Silver has two naturally occurring isotopes with the following isotopic masses: 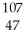 Ar
Ar 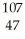 Ar 106.90509 108.9047
Ar 106.90509 108.9047
The average atomic mass of silver is 107.8682 amu.The fractional abundance of the lighter of the two isotopes is ________.
A)0.24221
B)0.48168
C)0.51835
D)0.75783
E)0.90474
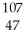 Ar
Ar 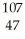 Ar 106.90509 108.9047
Ar 106.90509 108.9047The average atomic mass of silver is 107.8682 amu.The fractional abundance of the lighter of the two isotopes is ________.
A)0.24221
B)0.48168
C)0.51835
D)0.75783
E)0.90474

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
23
The nucleus of an atom does not contain ________.
A)protons
B)protons or neutrons
C)neutrons
D)subatomic particles
E)electrons
A)protons
B)protons or neutrons
C)neutrons
D)subatomic particles
E)electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
24
Different isotopes of a particular element contain the same number of ________.
A)protons
B)neutrons
C)protons and neutrons
D)protons, neutrons, and electrons
E)subatomic particles
A)protons
B)neutrons
C)protons and neutrons
D)protons, neutrons, and electrons
E)subatomic particles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
25
The atomic mass unit is presently based on assigning an exact integral mass (in amu)to an isotope of ________.
A)hydrogen
B)oxygen
C)sodium
D)carbon
E)helium
A)hydrogen
B)oxygen
C)sodium
D)carbon
E)helium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the symbol below,X = ________. 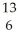 X
X
A)N
B)C
C)Al
D)K
E)not enough information to determine
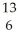 X
XA)N
B)C
C)Al
D)K
E)not enough information to determine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
27
In the symbol below,x is ________. 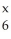 C
C
A)the number of neutrons
B)the atomic number
C)the mass number
D)the number of electrons
E)the elemental symbol
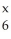 C
CA)the number of neutrons
B)the atomic number
C)the mass number
D)the number of electrons
E)the elemental symbol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
28
Gravitational forces act between objects in proportion to their ________.
A)volumes
B)masses
C)charges
D)polarizability
E)densities
A)volumes
B)masses
C)charges
D)polarizability
E)densities

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which one of the following basic forces is so small that it has no chemical significance?
A)weak nuclear force
B)strong nuclear force
C)electromagnetism
D)gravity
E)Coulomb's law
A)weak nuclear force
B)strong nuclear force
C)electromagnetism
D)gravity
E)Coulomb's law

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which combination of protons,neutrons,and electrons is correct for the isotope of copper, 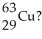
A)29 p+, 34 n°, 29 e-
B)29 p+, 29 n°, 63 e-
C)63 p+, 29 n°, 63 e-
D)34 p+, 29 n°, 34 e-
E)34 p+, 34 n°, 29 e-
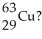
A)29 p+, 34 n°, 29 e-
B)29 p+, 29 n°, 63 e-
C)63 p+, 29 n°, 63 e-
D)34 p+, 29 n°, 34 e-
E)34 p+, 34 n°, 29 e-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which isotope has 36 electrons in an atom?
A)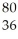 Kr
Kr
B)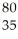 Br
Br
C)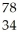 Se
Se
D)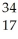 Cl
Cl
E)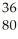 Hg
Hg
A)
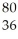 Kr
KrB)
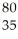 Br
BrC)
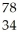 Se
SeD)
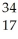 Cl
ClE)
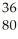 Hg
Hg
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which isotope has 45 neutrons?
A)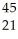 Sc
Sc
B)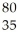 Br
Br
C)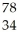 Se
Se
D)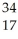 Cl
Cl
E)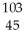 Rh
Rh
A)
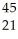 Sc
ScB)
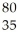 Br
BrC)
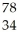 Se
SeD)
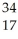 Cl
ClE)
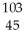 Rh
Rh
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
33
Different isotopes of a particular element contain different numbers of ________.
A)protons
B)neutrons
C)protons and neutrons
D)protons, neutrons, and electrons
E)None of the above is correct.
A)protons
B)neutrons
C)protons and neutrons
D)protons, neutrons, and electrons
E)None of the above is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following atoms has the smallest number of neutrons?
A)carbon-14
B)chlorine-35
C)carbon-12
D)carbon-13
E)bromine-79
A)carbon-14
B)chlorine-35
C)carbon-12
D)carbon-13
E)bromine-79

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
35
In the symbol below,x = ________. 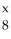 O
O
A)17
B)8
C)6
D)7
E)not enough information to determine
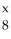 O
OA)17
B)8
C)6
D)7
E)not enough information to determine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
36
There are ________ electrons,________ protons,and ________ neutrons in an atom of 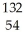 Xe.
Xe.
A)132, 132, 54
B)54, 54, 132
C)78, 78, 54
D)54, 54, 78
E)78, 78, 132
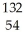 Xe.
Xe.A)132, 132, 54
B)54, 54, 132
C)78, 78, 54
D)54, 54, 78
E)78, 78, 132

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
37
The subatomic particles located in the nucleus with no overall charges are ________.
A)electrons
B)protons
C)neutrons
D)protons and neutrons
E)protons, neutrons, and electrons
A)electrons
B)protons
C)neutrons
D)protons and neutrons
E)protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
38
An atom of the most common isotope of gold, 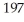 Au,has ________ protons,________ neutrons,and ________ electrons.
Au,has ________ protons,________ neutrons,and ________ electrons.
A)197, 79, 118
B)118, 79, 39
C)79, 197, 197
D)79, 118, 118
E)79, 118, 79
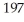 Au,has ________ protons,________ neutrons,and ________ electrons.
Au,has ________ protons,________ neutrons,and ________ electrons.A)197, 79, 118
B)118, 79, 39
C)79, 197, 197
D)79, 118, 118
E)79, 118, 79

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
39
In the symbol shown below,x = ________. 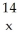 C
C
A)7
B)13
C)12
D)6
E)not enough information to determine
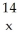 C
CA)7
B)13
C)12
D)6
E)not enough information to determine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
40
Isotopes are atoms that have the same ________ but differing ________.
A)atomic masses, charges
B)mass numbers, atomic numbers
C)atomic numbers, mass numbers
D)charges, atomic masses
E)mass numbers, charges
A)atomic masses, charges
B)mass numbers, atomic numbers
C)atomic numbers, mass numbers
D)charges, atomic masses
E)mass numbers, charges

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
41
Element X has three naturally occurring isotopes.The masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A)41.54
B)39.68
C)39.07
D)38.64
E)33.33

A)41.54
B)39.68
C)39.07
D)38.64
E)33.33

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
42
The element X has three naturally occurring isotopes.The isotopic masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A)33.33
B)55.74
C)56.11
D)57.23
E)56.29

A)33.33
B)55.74
C)56.11
D)57.23
E)56.29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which one of the following molecular formulas is also an empirical formula?
A)C6H6O2
B)C2H6SO
C)H2O2
D)H2P4O6
E)C6H6
A)C6H6O2
B)C2H6SO
C)H2O2
D)H2P4O6
E)C6H6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which pair of elements below should be the most similar in chemical properties?
A)C and O
B)B and As
C)I and Br
D)K and Kr
E)Cs and He
A)C and O
B)B and As
C)I and Br
D)K and Kr
E)Cs and He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
45
In the periodic table,the elements are arranged in ________.
A)alphabetical order
B)order of increasing atomic number
C)order of increasing metallic properties
D)order of increasing neutron content
E)increasing atomic mass
A)alphabetical order
B)order of increasing atomic number
C)order of increasing metallic properties
D)order of increasing neutron content
E)increasing atomic mass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
46
Vanadium has two naturally occurring isotopes,50V with an atomic mass of 49.9472 amu and 51V with an atomic mass of 50.9440.The atomic weight of vanadium is 50.9415.The percent abundances of the vanadium isotopes are ________% 50V and ________% 51V.
A)0.25, 99.75
B)99.75, 0.25
C)49, 51
D)1.0, 99
E)99, 1.0
A)0.25, 99.75
B)99.75, 0.25
C)49, 51
D)1.0, 99
E)99, 1.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
47
An element that appears in the lower left corner of the periodic table is ________.
A)either a metal or metalloid
B)definitely a metal
C)either a metalloid or a nonmetal
D)definitely a nonmetal
E)definitely a metalloid
A)either a metal or metalloid
B)definitely a metal
C)either a metalloid or a nonmetal
D)definitely a nonmetal
E)definitely a metalloid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
48
An unknown element is found to have three naturally occurring isotopes with atomic masses of 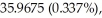
 and
and 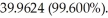 Which of the following is the unknown element?
Which of the following is the unknown element?
A)Ar
B)K
C)Cl
D)Ca
E)None of the above could be the unknown element.
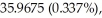
 and
and 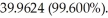 Which of the following is the unknown element?
Which of the following is the unknown element?A)Ar
B)K
C)Cl
D)Ca
E)None of the above could be the unknown element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
49
An element in the upper right corner of the periodic table ________.
A)is either a metal or metalloid
B)is definitely a metal
C)is either a metalloid or a nonmetal
D)is definitely a nonmetal
E)is definitely a metalloid
A)is either a metal or metalloid
B)is definitely a metal
C)is either a metalloid or a nonmetal
D)is definitely a nonmetal
E)is definitely a metalloid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
50
Elements in the same group of the periodic table typically have ________.
A)similar mass numbers
B)similar physical properties only
C)similar chemical properties only
D)similar atomic masses
E)similar physical and chemical properties
A)similar mass numbers
B)similar physical properties only
C)similar chemical properties only
D)similar atomic masses
E)similar physical and chemical properties

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
51
The element X has three naturally occurring isotopes.The masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 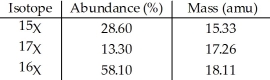
A)17.20
B)16.90
C)17.65
D)17.11
E)16. 90

A)17.20
B)16.90
C)17.65
D)17.11
E)16. 90

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties?
A)As, Br
B)Mg, Al
C)I, Br
D)Br, Kr
E)N, O
A)As, Br
B)Mg, Al
C)I, Br
D)Br, Kr
E)N, O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
53
Elements ________ exhibit similar physical and chemical properties.
A)with similar chemical symbols
B)with similar atomic masses
C)in the same period of the periodic table
D)on opposite sides of the periodic table
E)in the same group of the periodic table
A)with similar chemical symbols
B)with similar atomic masses
C)in the same period of the periodic table
D)on opposite sides of the periodic table
E)in the same group of the periodic table

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
54
The average atomic weight of copper,which has two naturally occurring isotopes,is 63.5.One of the isotopes has an atomic weight of 62.9 amu and constitutes 69.1% of the copper isotopes.The other isotope has an abundance of 30.9%.The atomic weight (amu)of the second isotope is ________ amu.
A)63.2
B)63.8
C)64.1
D)64.8
E)28.1
A)63.2
B)63.8
C)64.1
D)64.8
E)28.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
55
The element X has three naturally occurring isotopes.The isotopic masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A)161.75
B)162.03
C)162.35
D)163.15
E)33.33

A)161.75
B)162.03
C)162.35
D)163.15
E)33.33

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
56
The element X has two naturally occurring isotopes.The masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A)30.20
B)33.20
C)34.02
D)35.22
E)32.73

A)30.20
B)33.20
C)34.02
D)35.22
E)32.73

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
57
The elements in groups 1A,6A,and 7A are called ________,respectively.
A)alkaline earth metals, halogens, and chalcogens
B)alkali metals, chalcogens, and halogens
C)alkali metals, halogens, and noble gases
D)alkaline earth metals, transition metals, and halogens
E)halogens, transition metals, and alkali metals
A)alkaline earth metals, halogens, and chalcogens
B)alkali metals, chalcogens, and halogens
C)alkali metals, halogens, and noble gases
D)alkaline earth metals, transition metals, and halogens
E)halogens, transition metals, and alkali metals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties?
A)H, Li
B)Cs, Ba
C)Ca, Sr
D)Ga, Ge
E)C, O
A)H, Li
B)Cs, Ba
C)Ca, Sr
D)Ga, Ge
E)C, O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
59
The element X has three naturally occurring isotopes.The masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A)219.7
B)220.4
C)220.42
D)218.5
E)221.0

A)219.7
B)220.4
C)220.42
D)218.5
E)221.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties?
A)O, S
B)C, N
C)K, Ca
D)H, He
E)Si, P
A)O, S
B)C, N
C)K, Ca
D)H, He
E)Si, P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
61
There are ________ protons,________ neutrons,and ________ electrons in 238U+5.
A)146, 92, 92
B)92, 146, 87
C)92, 146, 92
D)92, 92, 87
E)146, 92, 97
A)146, 92, 92
B)92, 146, 87
C)92, 146, 92
D)92, 92, 87
E)146, 92, 97

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which species has 18 electrons?
A)(39K)
B)(32S2-)
C)(35Cl)
D)(27Al3+)
E)(45Sc3+)
A)(39K)
B)(32S2-)
C)(35Cl)
D)(27Al3+)
E)(45Sc3+)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which one of the following species has as many electrons as it has neutrons?
A)(1H)
B)(40Ca2+)
C)(14C)
D)(19F-)
E)(14C2+)
A)(1H)
B)(40Ca2+)
C)(14C)
D)(19F-)
E)(14C2+)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
64
Of the choices below,which one is not an ionic compound?
A)PCl5
B)MoCl6
C)RbCl
D)PbCl2
E)NaCl
A)PCl5
B)MoCl6
C)RbCl
D)PbCl2
E)NaCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
65
The molecular formula of a compound is always ________ the empirical formula.
A)more complex than
B)different from
C)an integral multiple of
D)the same as
E)simpler than
A)more complex than
B)different from
C)an integral multiple of
D)the same as
E)simpler than

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
66
An empirical formula always indicates ________.
A)which atoms are attached to which in a molecule
B)how many of each atom are in a molecule
C)the simplest whole-number ratio of different atoms in a compound
D)the isotope of each element in a compound
E)the geometry of a molecule
A)which atoms are attached to which in a molecule
B)how many of each atom are in a molecule
C)the simplest whole-number ratio of different atoms in a compound
D)the isotope of each element in a compound
E)the geometry of a molecule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
67
There are ________ protons,________ neutrons,and ________ electrons in 131I-.
A)131, 53, 54
B)131, 53, 52
C)53, 78, 54
D)53, 131, 52
E)53, 78, 52
A)131, 53, 54
B)131, 53, 52
C)53, 78, 54
D)53, 131, 52
E)53, 78, 52

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which species has 16 protons?
A)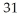 P
P
B)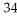
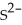
C)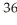 Cl
Cl
D)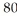 B
B 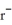
E)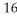 O
O
A)
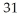 P
PB)
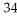
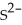
C)
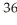 Cl
ClD)
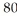 B
B 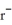
E)
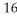 O
O
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
69
Of the following,________ contains the greatest number of electrons.
A)P3+
B)P
C)P2-
D)P3-
E)P2+
A)P3+
B)P
C)P2-
D)P3-
E)P2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which pair of elements is most apt to form a molecular compound with each other?
A)aluminum, oxygen
B)magnesium, iodine
C)sulfur, fluorine
D)potassium, lithium
E)barium, bromine
A)aluminum, oxygen
B)magnesium, iodine
C)sulfur, fluorine
D)potassium, lithium
E)barium, bromine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following species is an isotope of 79Br?
A)(40Ar+)
B)(34S2-)
C)(79Br-)
D)(80Br)
E)(79Se)
A)(40Ar+)
B)(34S2-)
C)(79Br-)
D)(80Br)
E)(79Se)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the following species contains 18 electrons?
A)(31P)
B)(34S2-)
C)(36Cl)
D)(80Br-)
E)(16O)
A)(31P)
B)(34S2-)
C)(36Cl)
D)(80Br-)
E)(16O)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which pair of elements is most apt to form an ionic compound with each other?
A)barium, bromine
B)calcium, sodium
C)oxygen, fluorine
D)sulfur, fluorine
E)nitrogen, hydrogen
A)barium, bromine
B)calcium, sodium
C)oxygen, fluorine
D)sulfur, fluorine
E)nitrogen, hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
74
A molecular formula always indicates ________.
A)how many of each atom are in a molecule
B)the simplest whole-number ratio of different atoms in a compound
C)which atoms are attached to which in a molecule
D)the isotope of each element in a compound
E)the geometry of a molecule
A)how many of each atom are in a molecule
B)the simplest whole-number ratio of different atoms in a compound
C)which atoms are attached to which in a molecule
D)the isotope of each element in a compound
E)the geometry of a molecule

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which species has 54 electrons?
A)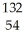 Xe+
Xe+
B)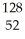 Te2-
Te2-
C)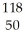 Sn4+
Sn4+
D)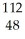 Cd
Cd
E)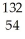 Xe2+
Xe2+
A)
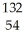 Xe+
Xe+B)
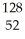 Te2-
Te2-C)
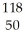 Sn4+
Sn4+D)
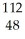 Cd
CdE)
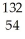 Xe2+
Xe2+
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
76
Formulas that show how atoms are attached in a molecule are called ________.
A)molecular formulas
B)ionic formulas
C)empirical formulas
D)diatomic formulas
E)structural formulas
A)molecular formulas
B)ionic formulas
C)empirical formulas
D)diatomic formulas
E)structural formulas

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which compounds do not have the same empirical formula?
A)C2H2, C6H6
B)CO, CO2
C)C2H4, C3H6
D)C2H4O2, C6H12O6
E)C2H5COOCH3, CH3CHO
A)C2H2, C6H6
B)CO, CO2
C)C2H4, C3H6
D)C2H4O2, C6H12O6
E)C2H5COOCH3, CH3CHO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which type of formula provides the most information about a compound?
A)empirical
B)molecular
C)simplest
D)structural
E)chemical
A)empirical
B)molecular
C)simplest
D)structural
E)chemical

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which of the following compounds would you expect to be ionic?
A)H2O
B)CO2
C)SrCl2
D)SO2
E)H2S
A)H2O
B)CO2
C)SrCl2
D)SO2
E)H2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which species contains 68 neutrons?
A)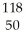 Sn+2
Sn+2
B)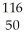 Sn+2
Sn+2
C)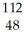 Cd+2
Cd+2
D)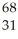 Ga
Ga
E)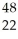 Ti
Ti
A)
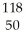 Sn+2
Sn+2B)
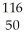 Sn+2
Sn+2C)
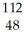 Cd+2
Cd+2D)
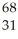 Ga
GaE)
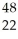 Ti
Ti
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 249 في هذه المجموعة.
فتح الحزمة
k this deck








