Deck 7: Quantitative Composition of Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/111
العب
ملء الشاشة (f)
Deck 7: Quantitative Composition of Compounds
1
What is the mass of 1.36 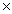 1022 molecules of HCl?
1022 molecules of HCl?
A)0.824g
B)1610g
C)36.5g
D)136g
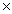 1022 molecules of HCl?
1022 molecules of HCl?A)0.824g
B)1610g
C)36.5g
D)136g
0.824g
2
How many molecules are present in 0.340g of HCl?
A)1.78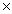 10-22
10-22
B)6.46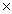 1025
1025
C)5.61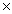 1021
1021
D)2.06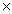 10-23
10-23
A)1.78
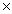 10-22
10-22B)6.46
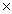 1025
1025C)5.61
 1021
1021D)2.06
 10-23
10-235.61  1021
1021
 1021
1021 3
How many moles of chloride ions are present in 18.4g of CaCl2?
A)0.332 moles
B)0.166 moles
C)6.03 moles
D)184 moles
A)0.332 moles
B)0.166 moles
C)6.03 moles
D)184 moles
0.332 moles
4
How many moles are present in 140.g of CaCl2?
A)15500 moles
B)1.46 moles
C)1.26 moles
D)0.793 moles
A)15500 moles
B)1.46 moles
C)1.26 moles
D)0.793 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the mass of 1.80 moles of MgSO4?
A)1.80g
B)10.8g
C)217g
D)1.08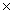 1024 g
1024 g
A)1.80g
B)10.8g
C)217g
D)1.08
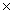 1024 g
1024 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the mass of 4.36 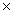 1023 molecules of CCl4?
1023 molecules of CCl4?
A)312g
B)212g
C)111g
D)154g
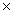 1023 molecules of CCl4?
1023 molecules of CCl4?A)312g
B)212g
C)111g
D)154g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
7
How many moles are present in a sample of HCl with a mass of 3.65g?
A)3.46 moles
B)0.100 moles
C)2.19 1024 moles
1024 moles
D)10.0 moles
A)3.46 moles
B)0.100 moles
C)2.19
 1024 moles
1024 molesD)10.0 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the mass of one mole of HCl?
A)1.01g
B)6 1023 g
1023 g
C)35.45g
D)36.46g
A)1.01g
B)6
 1023 g
1023 gC)35.45g
D)36.46g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
9
How many units of AgNO3 are present in 147g of this compound?
A)4.15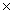 10-20
10-20
B)5.21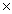 1023
1023
C)1.47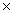 1021
1021
D)6.96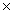 1023
1023
A)4.15
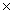 10-20
10-20B)5.21
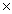 1023
1023C)1.47
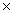 1021
1021D)6.96
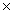 1023
1023
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many O atoms are present in 1.04 moles of MgSO4?
A)1.04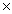 1023 atoms
1023 atoms
B)4.16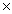 1023 atoms
1023 atoms
C)2.51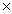 1024 atoms
1024 atoms
D)6.26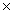 1023 atoms
1023 atoms
A)1.04
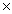 1023 atoms
1023 atomsB)4.16
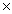 1023 atoms
1023 atomsC)2.51
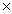 1024 atoms
1024 atomsD)6.26
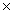 1023 atoms
1023 atoms
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many molecules are present in 146g of HCl?
A)3.20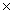 1027
1027
B)8.84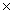 10-21
10-21
C)2.41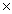 1024
1024
D)1.50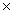 1023
1023
A)3.20
 1027
1027B)8.84
 10-21
10-21C)2.41
 1024
1024D)1.50
 1023
1023
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many chloride ions are present in 347g of CaCl2?
A)1.88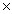 1024
1024
B)1.93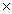 1023
1023
C)3.77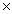 1024
1024
D)6.40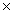 10-20
10-20
A)1.88
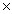 1024
1024B)1.93
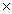 1023
1023C)3.77
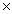 1024
1024D)6.40
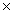 10-20
10-20
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the mass of 0.118 moles of AgNO3?
A)0.118 g
B)0.710g
C)7.10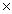 1023 g.
1023 g.
D)20.0g
A)0.118 g
B)0.710g
C)7.10
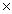 1023 g.
1023 g.D)20.0g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
14
How many units of MgSO4 are present in 18.0g of this compound?
A)6.02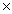 1023
1023
B)3.60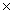 10-21
10-21
C)4.03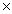 1024
1024
D)9.00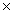 1022
1022
A)6.02
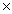 1023
1023B)3.60
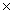 10-21
10-21C)4.03
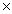 1024
1024D)9.00
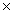 1022
1022
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the mass of 0.360 moles of HCl?
A)0.360g
B)13.1g
C)101g
D)2.17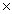 1023 g
1023 g
A)0.360g
B)13.1g
C)101g
D)2.17
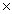 1023 g
1023 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
16
How many units of AgNO3 are present in 1.36g of this compound?
A)3.84 X 10-22
B)4.82 X 10 21
C)1.36 X 1023
D)7.52 X 1025
A)3.84 X 10-22
B)4.82 X 10 21
C)1.36 X 1023
D)7.52 X 1025

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
17
How many moles of silver ions are present in 32.46g of AgNO3?
A)0.191 moles
B)5520 moles
C)5.23 moles
D)32.5 moles
A)0.191 moles
B)5520 moles
C)5.23 moles
D)32.5 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
18
How many moles are present in 0.140g of HCl?
A)0.140 moles
B)0.00384 moles
C)1.40 moles
D)5.01 moles
A)0.140 moles
B)0.00384 moles
C)1.40 moles
D)5.01 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many moles are present in 1.64g of MgSO4?
A)0.734 moles
B)1.64 moles
C)120.Moles
D)0.0136 moles
A)0.734 moles
B)1.64 moles
C)120.Moles
D)0.0136 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the mass of 3.61 moles of CaCl2?
A)3.61g
B)21.7g
C)21.7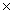 1023 g
1023 g
D)401g
A)3.61g
B)21.7g
C)21.7
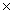 1023 g
1023 gD)401g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
21
How many molecules are present in 1.30 moles of CCl4?
A)2.00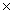 1025
1025
B)7.83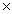 1023
1023
C)1.30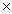 1023
1023
D)7.83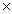 1024
1024
A)2.00
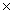 1025
1025B)7.83
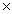 1023
1023C)1.30
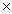 1023
1023D)7.83
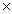 1024
1024
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
22
How many molecules are present in 0.500 moles of CH4?
A)3.01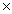 1023
1023
B)6.02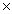 1023
1023
C)8.00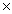 1023
1023
D)5.00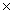 1022
1022
A)3.01
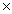 1023
1023B)6.02
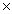 1023
1023C)8.00
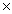 1023
1023D)5.00
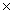 1022
1022
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
23
How many chlorine atoms are present in 0.778 moles of Cl2?
A)4.69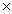 1023
1023
B)9.37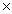 1023
1023
C)2.34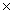 1023
1023
D)1.56
A)4.69
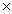 1023
1023B)9.37
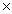 1023
1023C)2.34
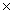 1023
1023D)1.56

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many molecules are present in 4.21 moles of HBr?
A)1.53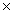 1025
1025
B)2.53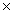 1025
1025
C)4.21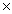 1023
1023
D)2.53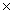 1024
1024
A)1.53
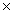 1025
1025B)2.53
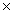 1025
1025C)4.21
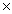 1023
1023D)2.53
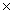 1024
1024
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
25
How many moles of hydrogen atoms are present in 2.41 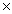 1024 molecules of CH4?
1024 molecules of CH4?
A)0.250
B)4.00
C)1.00
D)16.0
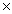 1024 molecules of CH4?
1024 molecules of CH4?A)0.250
B)4.00
C)1.00
D)16.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many moles are present in 1.51 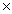 1023 molecules of HCl?
1023 molecules of HCl?
A)9.12
B)0.251
C)3.99
D)146
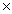 1023 molecules of HCl?
1023 molecules of HCl?A)9.12
B)0.251
C)3.99
D)146

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
27
How many moles are present in 6.02  1024 molecules of I2?
1024 molecules of I2?
A)0.100
B)1.00
C)10.0
D)100.
 1024 molecules of I2?
1024 molecules of I2?A)0.100
B)1.00
C)10.0
D)100.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the molar mass of KClO3?
A)90.55g
B)122.55g
C)161.45g
D)6.02 1023 g
1023 g
A)90.55g
B)122.55g
C)161.45g
D)6.02
 1023 g
1023 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the mass of 3.06 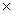 1022 molecules of CO2?
1022 molecules of CO2?
A)135g
B)2.24g
C)14.4g
D)866g
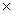 1022 molecules of CO2?
1022 molecules of CO2?A)135g
B)2.24g
C)14.4g
D)866g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
30
How many moles are present in 1.21 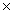 1024 molecules of HBr?
1024 molecules of HBr?
A)7.28
B)0.500
C)2.01
D)0.201
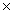 1024 molecules of HBr?
1024 molecules of HBr?A)7.28
B)0.500
C)2.01
D)0.201

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
31
How many moles are present in 6.02 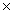 1022 molecules of I2?
1022 molecules of I2?
A)0.100
B)1.00
C)10.0
D)100.
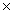 1022 molecules of I2?
1022 molecules of I2?A)0.100
B)1.00
C)10.0
D)100.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the mass of 2.00 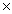 1023 molecules of H2O?
1023 molecules of H2O?
A)5.99g
B)54.2g
C)18.0g
D)36.0g
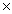 1023 molecules of H2O?
1023 molecules of H2O?A)5.99g
B)54.2g
C)18.0g
D)36.0g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the mass of 1.20 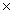 1023 molecules of CH3OH?
1023 molecules of CH3OH?
A)161g
B)38.5g
C)6.39g
D)32.1g
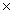 1023 molecules of CH3OH?
1023 molecules of CH3OH?A)161g
B)38.5g
C)6.39g
D)32.1g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the molar mass of sodium chloride?
A)6.02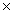 1023 g
1023 g
B)1.00g
C)67.53g
D)58.44g
A)6.02
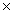 1023 g
1023 gB)1.00g
C)67.53g
D)58.44g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the molar mass of Mg(ClO4)2?
A)223.21g
B)1.00g
C)301.01g
D)187.76g
A)223.21g
B)1.00g
C)301.01g
D)187.76g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
36
How many molecules of CH3OH will contain 3.20 x 1023 H atoms?
A)3.20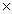 1023
1023
B)1.07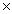 1023
1023
C)8.00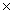 1022
1022
D)1.28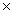 1024
1024
A)3.20
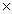 1023
1023B)1.07
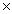 1023
1023C)8.00
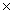 1022
1022D)1.28
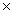 1024
1024
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many molecules are present in 0.300 moles of HI?
A)2.43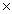 1024
1024
B)1.81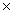 1023
1023
C)2.01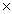 1024
1024
D)3.00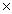 1022
1022
A)2.43
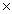 1024
1024B)1.81
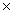 1023
1023C)2.01
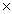 1024
1024D)3.00
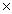 1022
1022
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the mass of 3.01 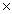 1024 molecules of Cl2?
1024 molecules of Cl2?
A)137g
B)14.2g
C)213g
D)355g
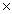 1024 molecules of Cl2?
1024 molecules of Cl2?A)137g
B)14.2g
C)213g
D)355g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
39
How many moles are present in 3.01 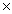 1023 molecules of CH4?
1023 molecules of CH4?
A)48.0
B)0.500
C)8.00
D)2.00
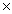 1023 molecules of CH4?
1023 molecules of CH4?A)48.0
B)0.500
C)8.00
D)2.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many molecules are present in 4.00 moles of Cl2?
A)1.51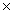 1023
1023
B)2.41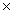 1024
1024
C)7.09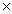 1024
1024
D)2.84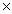 1025
1025
A)1.51
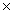 1023
1023B)2.41
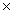 1024
1024C)7.09
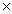 1024
1024D)2.84
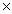 1025
1025
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the percent by mass of hydrogen in NaHCO3?
A)27.4%
B)1.20%
C)24.7%
D)44.2%
A)27.4%
B)1.20%
C)24.7%
D)44.2%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
42
A compound is found to consist of 1.37g of iron and 2.62g of chlorine.What is the percent by mass of chlorine in the compound?
A)34.3%
B)65.7%
C)52.3%
D)87.6%
A)34.3%
B)65.7%
C)52.3%
D)87.6%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
43
A substance is known to contain 2.00 g of carbon for every 5.33 g of oxygen.How many grams of carbon will be present in 55.0 g of this substance?
A)15.0 g
B)27.5 g
C)20.6 g
D)10.3 g
A)15.0 g
B)27.5 g
C)20.6 g
D)10.3 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the percent by mass of chlorine in Ca(ClO3)2?
A)20.7%
B)34.3%
C)38.7%
D)22.8%
A)20.7%
B)34.3%
C)38.7%
D)22.8%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many grams of carbon are present in 45.0 g of CCl4?
A)0.293 g
B)3.51 g
C)5.76 g
D)41.49 g
A)0.293 g
B)3.51 g
C)5.76 g
D)41.49 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the molar mass of CaSO4?
A)88.14g
B)136.14g
C)184.30g
D)232.32g
A)88.14g
B)136.14g
C)184.30g
D)232.32g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
47
A compound is found to consist of 2.89g of calcium and 5.11g of chlorine.What is the percent by mass of calcium in the compound?
A)56.6%
B)36.1%
C)63.9%
D)100%
A)56.6%
B)36.1%
C)63.9%
D)100%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
48
A 6.00g sample of calcium sulfide is found to contain 3.33g of calcium.What is the percent by mass of sulfur in the compound?
A)80.2%
B)35.7%
C)44.5%
D)28.6%
A)80.2%
B)35.7%
C)44.5%
D)28.6%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many grams of oxygen are present in 76.8 g of CO2?
A)1.75 g
B)55.8 g
C)27.9 g
D)48.9 g
A)1.75 g
B)55.8 g
C)27.9 g
D)48.9 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
50
A substance is found to consist of 1.900g of silver,0.250g of nitrogen,and 0.850g of oxygen.What is the percent by mass of oxygen in the compound?
A)28.3%
B)8.33%
C)63.3%
D)85.0%
A)28.3%
B)8.33%
C)63.3%
D)85.0%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the empirical formula of a substance that consists of 85.60% carbon and 14.40% hydrogen?
A)CH
B)CH2
C)CH3
D)CH4
A)CH
B)CH2
C)CH3
D)CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the percent by mass of hydrogen in sulfuric acid?
A)1.04%
B)2.06%
C)4.03%
D)5.93%
A)1.04%
B)2.06%
C)4.03%
D)5.93%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
53
A 10.00g sample of a substance is found to contain 5.12g of water.What is the percent by mass of water in the compound?
A)5.12%
B)4.88%
C)48.8%
D)51.2%
A)5.12%
B)4.88%
C)48.8%
D)51.2%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
54
A 5.00g sample of potassium chloride is found to contain 2.62g of potassium.What is the percent by mass of potassium in the compound?
A)90.8%
B)47.6%
C)52.4%
D)34.4%
A)90.8%
B)47.6%
C)52.4%
D)34.4%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the molar mass of Sr(NO3)2?
A)117.63g
B)181.63g
C)237.67g
D)211.64g
A)117.63g
B)181.63g
C)237.67g
D)211.64g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the percent by mass of carbon in carbon dioxide?
A)3.66%
B)366%
C)27.28%
D)42.9%
A)3.66%
B)366%
C)27.28%
D)42.9%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
57
A compound is known to consist of 19.36% carbon,3.25% hydrogen,and the rest is oxygen.How many grams of this compound will be needed in order to have 23.5 g of oxygen?
A)30.4 g
B)104 g
C)80.4 g
D)53.1 g
A)30.4 g
B)104 g
C)80.4 g
D)53.1 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the molar mass of carbon tetrachloride?
A)47.46g
B)82.91g
C)118.36g
D)153.81g
A)47.46g
B)82.91g
C)118.36g
D)153.81g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the percent by mass of oxygen in water?
A)11.2%
B)88.8%
C)5.94%
D)94.1%
A)11.2%
B)88.8%
C)5.94%
D)94.1%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the percent by mass of hydrogen in HC2H3O2?
A)1.68%
B)5.05%
C)6.73%
D)53.3%
A)1.68%
B)5.05%
C)6.73%
D)53.3%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
61
A 65.2 g sample of a compound contains 32.1 g of phosphorus and the rest is oxygen.What is the empirical formula of the compound?
A)P3O2
B)PO2
C)PO
D)P2O3
A)P3O2
B)PO2
C)PO
D)P2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the molecular formula of a compound with the empirical formula CH and molar mass of 26.04g?
A)CH
B)C2H
C)CH2
D)C2H2
A)CH
B)C2H
C)CH2
D)C2H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the empirical formula of a substance that consists of 3.704g aluminum and 3.295g oxygen?
A)AlO
B)AlO2
C)Al2O3
D)Al3O2
A)AlO
B)AlO2
C)Al2O3
D)Al3O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the empirical formula of a substance that consists of 32.86% potassium and 67.14% bromine?
A)KBr
B)K2Br
C)KBr2
D)K2Br3
A)KBr
B)K2Br
C)KBr2
D)K2Br3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the empirical formula of a substance that consists of 0.910g calcium,0.636g nitrogen,and 1.453g oxygen?
A)CaNO
B)Ca2NO7
C)CaN2O4
D)CaN2O6
A)CaNO
B)Ca2NO7
C)CaN2O4
D)CaN2O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which statement is incorrect?
A)One mole contains 6.02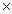 1023 molecules.
1023 molecules.
B)One molar mass equals one mole.
C)One mole equals 6.02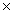 1023 g of a compound.
1023 g of a compound.
D)One mole of water contains the same number of molecules as one mole of carbon dioxide.
A)One mole contains 6.02
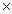 1023 molecules.
1023 molecules.B)One molar mass equals one mole.
C)One mole equals 6.02
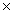 1023 g of a compound.
1023 g of a compound.D)One mole of water contains the same number of molecules as one mole of carbon dioxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
67
All the following contain the same number of molecules except
A)64.06g of sulfur dioxide molecules
B)One mole of water molecules
C)88.02g of carbon dioxide molecules
D)2.02g of hydrogen molecules
A)64.06g of sulfur dioxide molecules
B)One mole of water molecules
C)88.02g of carbon dioxide molecules
D)2.02g of hydrogen molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the molecular formula of a compound with the empirical formula CH2O and molar mass of 180.18g?
A)CH2O
B)C2H4O2
C)C4H8O4
D)C6H12O6
A)CH2O
B)C2H4O2
C)C4H8O4
D)C6H12O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the molecular formula of a compound with the empirical formula C13H19O2 and molar mass of 414.64g?
A)CHO
B)C13H19O2
C)C26H38O4
D)C39H57O6
A)CHO
B)C13H19O2
C)C26H38O4
D)C39H57O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
70
In which pair would both compounds have the same empirical formula?
A)H2O and H2O2
B)BaSO4 and BaSO3
C)FeO and Fe2O3
D)C6H12O6 and HC2H3O2
A)H2O and H2O2
B)BaSO4 and BaSO3
C)FeO and Fe2O3
D)C6H12O6 and HC2H3O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
71
What is the empirical formula of a compound that consists of 39.99% carbon,6.73% hydrogen,and 53.28% oxygen?
A)CHO
B)C2HO
C)CH2O
D)CHO2
A)CHO
B)C2HO
C)CH2O
D)CHO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the molecular formula of a compound with the empirical formula CH2Cl and molar mass of 98.96g?
A)CH2Cl
B)C2H4Cl2
C)C3H6Cl3
D)C4H8Cl4
A)CH2Cl
B)C2H4Cl2
C)C3H6Cl3
D)C4H8Cl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
73
One atom of sulfur has a mass of
A)5.33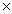 10-23 g
10-23 g
B)32.06g
C)1.88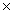 1022 g
1022 g
D)1.93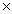 1025 g
1025 g
A)5.33
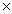 10-23 g
10-23 gB)32.06g
C)1.88
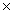 1022 g
1022 gD)1.93
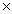 1025 g
1025 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
74
In which pair would both compounds have the same empirical formula?
A)C2H4 and C3H6
B)K2CrO4 and K2Cr2O7
C)NaHCO3 and Na2CO3
D)FeCl3 and FeCl2
A)C2H4 and C3H6
B)K2CrO4 and K2Cr2O7
C)NaHCO3 and Na2CO3
D)FeCl3 and FeCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
75
A 30.5 g sample of a compound contains 9.29 g of nitrogen and the rest is oxygen.What is the empirical formula of the compound?
A)N2O3
B)N2O
C)NO
D)NO2
A)N2O3
B)N2O
C)NO
D)NO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
76
What is the molecular formula of a compound with the empirical formula CHO2 and molar mass of 90.04g?
A)CHO2
B)C2H2O4
C)C3H3O6
D)C4H4O8
A)CHO2
B)C2H2O4
C)C3H3O6
D)C4H4O8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the empirical formula of a compound that consists of 2.424g magnesium,3.197g sulfur,and 6.380g oxygen?
A)MgSO
B)Mg2SO4
C)MgSO4
D)MgSO3
A)MgSO
B)Mg2SO4
C)MgSO4
D)MgSO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
78
A compound consists of 4.50 g of nitrogen for every 7.71 g of oxygen and has the same empirical and molecular formula.Identify the compound.
A)NO2
B)N2O
C)N2O3
D)NO3
A)NO2
B)N2O
C)N2O3
D)NO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
79
One atom of magnesium has a mass of
A)24.31g
B)4.04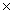 10-23 g
10-23 g
C)2.48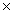 1022 g
1022 g
D)6.02 1023 g
1023 g
A)24.31g
B)4.04
 10-23 g
10-23 gC)2.48
 1022 g
1022 gD)6.02
 1023 g
1023 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the molecular formula of a compound with the empirical formula CH2O and molar mass of 60.06g?
A)CH2O
B)C2H4O2
C)C3H6O3
D)C4H8O4
A)CH2O
B)C2H4O2
C)C3H6O3
D)C4H8O4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck








