Deck 14: Solutions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/100
العب
ملء الشاشة (f)
Deck 14: Solutions
1
At which temperature would CO2 gas be most soluble?
A)10. ° C
B)20. ° C
C)30. ° C
D)40. °C
A)10. ° C
B)20. ° C
C)30. ° C
D)40. °C
10. ° C
2
As solute surface area increases,the rate of dissolving
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.
increases.
3
Use the figure below to determine the mass of potassium chloride required to prepare a saturated solution in 50.0 mL of water at 40°C. 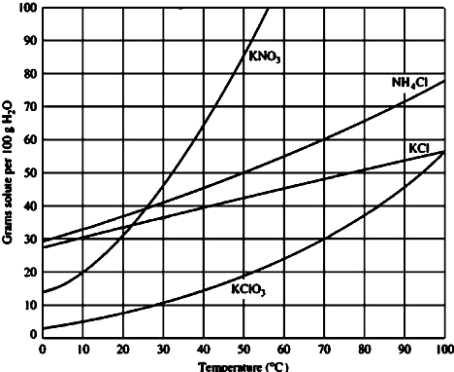
A)40 g
B)20 g
C)10 g
D)45 g

A)40 g
B)20 g
C)10 g
D)45 g
20 g
4
The dissolved solute is in equilibrium with undissolved solute in a solution which is
A)saturated.
B)unsaturated.
C)supersaturated.
A)saturated.
B)unsaturated.
C)supersaturated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
5
As pressure increases,the solubility of a gas in water
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
6
A solution containing 50.0 g of ammonium chloride in 100.0 mL of water at 60°C is 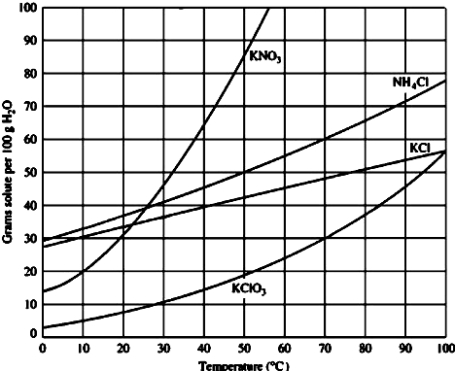
A)saturated.
B)unsaturated.
C)supersaturated.

A)saturated.
B)unsaturated.
C)supersaturated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
7
The solubility of ammonium chloride at 70. ° C is 60.g of solute per 100.g of water.Whichsolution would be unsaturated at 70. ° C?
A)110.g of solute per 200.g of water
B)120.g of solute per 200.g of water
C)30.g of solute per 50.g of water
D)35g of solute per 50.g of water
A)110.g of solute per 200.g of water
B)120.g of solute per 200.g of water
C)30.g of solute per 50.g of water
D)35g of solute per 50.g of water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
8
At which temperature would KCl solid be most soluble?
A)10. ° C
B)20. ° C
C)30. ° C
D)40. °C
A)10. ° C
B)20. ° C
C)30. ° C
D)40. °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
9
At which temperature would KNO3 solid be most soluble?
A)283 K
B)293 K
C)303 K
D)313 K
A)283 K
B)293 K
C)303 K
D)313 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which phase of matter can act as a solvent?
A)Solid
B)Liquid
C)Gas
D)All the above
A)Solid
B)Liquid
C)Gas
D)All the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
11
As pressure increases,the solubility of a solid in water
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
12
At which temperature would NH3 gas be most soluble?
A)283 K
B)293 K
C)303 K
D)313 K
A)283 K
B)293 K
C)303 K
D)313 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
13
The solubility of ammonium chloride at 70. °C is 60.g of solute per 100.g of water.Which solution would be saturated at 70. ° C?
A)60.g of solute in 200.g of water
B)90.g of solute in 200.g of water
C)30.g of solute in 50.g of water
D)35g of solute in 50.g of water
A)60.g of solute in 200.g of water
B)90.g of solute in 200.g of water
C)30.g of solute in 50.g of water
D)35g of solute in 50.g of water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
14
As temperature increases,the solubility of a gas in water
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
15
As temperature increases,the solubility of most solids in water
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
16
At which pressure would nitrogen gas be most soluble?
A)1.0 atm
B)1.5 atm
C)2.0 atm
D)2.5 atm
A)1.0 atm
B)1.5 atm
C)2.0 atm
D)2.5 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
17
At which pressure would carbon dioxide gas be most soluble?
A)600 torr
B)700 torr
C)800 torr
D)900 torr
A)600 torr
B)700 torr
C)800 torr
D)900 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
18
A solution is a
A)homogeneous compound.
B)heterogeneous compound.
C)homogeneous mixture.
D)heterogeneous mixture.
A)homogeneous compound.
B)heterogeneous compound.
C)homogeneous mixture.
D)heterogeneous mixture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which liquid is miscible with water?
A)Carbon tetrachloride
B)Ethyl alcohol
C)Bromine
D)Oil
A)Carbon tetrachloride
B)Ethyl alcohol
C)Bromine
D)Oil

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
20
As solute particle size increases,the rate of dissolving
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
21
One liter of 2.0 M KCl solution and two liters of 1.0 M KCl solution have the same
A)Density
B)Concentration
C)Volume
D)Moles of solute
A)Density
B)Concentration
C)Volume
D)Moles of solute

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which salt is most soluble in water?
A)Chromium(III)nitrate
B)Calcium carbonate
C)Silver iodide
D)Barium phosphate
A)Chromium(III)nitrate
B)Calcium carbonate
C)Silver iodide
D)Barium phosphate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which one molar solution would have the highest freezing point?
A)KCl
B)BaCl2
C)AlCl3
D)CH3OH
A)KCl
B)BaCl2
C)AlCl3
D)CH3OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
24
For which of the following solutes would dissolving 40 g in 100 g of water will result in an unsaturated solution at 30°C? 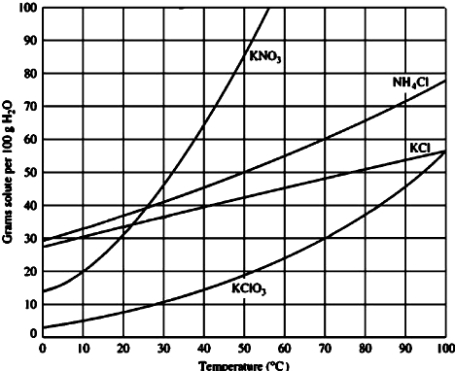
A)KNO3
B)NH4Cl
C)KCl
D)KClO3

A)KNO3
B)NH4Cl
C)KCl
D)KClO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following solutions would have the lowest boiling point?
A)1.0 g of NaCl in 100 g of water
B)1.0 g of KNO3 in 100 g of water
C)1.0 g of NaC2H3O2 in 100 g of water
D)All the solutions will have the same boiling point.
A)1.0 g of NaCl in 100 g of water
B)1.0 g of KNO3 in 100 g of water
C)1.0 g of NaC2H3O2 in 100 g of water
D)All the solutions will have the same boiling point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following solutions will have the highest boiling point?
A)1.0 g of NaCl in 100 g of water
B)1.0 g of KNO3 in 100 g of water
C)1.0 g of NaC2H3O2 in 100 g of water
D)All the solutions will have the same boiling point.
A)1.0 g of NaCl in 100 g of water
B)1.0 g of KNO3 in 100 g of water
C)1.0 g of NaC2H3O2 in 100 g of water
D)All the solutions will have the same boiling point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which would most likely increase the solubility of a solid in water?
A)Stirring
B)Increase the surface area of the solid
C)Increase the pressure
D)Increase the temperature
A)Stirring
B)Increase the surface area of the solid
C)Increase the pressure
D)Increase the temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following solutions will have the lowest freezing point?
A)2.5 g of KCl in 100 g of water
B)2.5 g of NH4Cl in 100 g of water
C)2.5 g of NaOH in 100 g of water
D)All the solutions will have the same freezing point.
A)2.5 g of KCl in 100 g of water
B)2.5 g of NH4Cl in 100 g of water
C)2.5 g of NaOH in 100 g of water
D)All the solutions will have the same freezing point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the mass percent of the solution formed when 5.0 g of solute is dissolved in 40.g of water?
A)8.0 %
B)11 %
C)13 %
D)14 %
A)8.0 %
B)11 %
C)13 %
D)14 %

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
30
The normal boiling point of pure water is 373 K.As a solute dissolves in water,the boiling point of the solution will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
31
The addition of a crystal of sodium chlorate to a sodium chlorate solution causes additional crystals of sodium chlorate to precipitate.The original solution was
A)saturated.
B)supersaturated.
C)snsaturated.
A)saturated.
B)supersaturated.
C)snsaturated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
32
What masses of KBr and water are needed to produce 300.g of a solution that is 3.00 % bymass?
A)9.00 g of KBr and 300.g of water
B)9.00 g of KBr and 291 g of water
C)300.g of KBr and 9.00 g of water
D)291 g of KBr and 9.00 g of water
A)9.00 g of KBr and 300.g of water
B)9.00 g of KBr and 291 g of water
C)300.g of KBr and 9.00 g of water
D)291 g of KBr and 9.00 g of water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the mass percent of a solution formed by dissolving 50.0 g of glucose in 1000.g of water?
A)4.76 %
B)5.00 %
C)5.26 %
D)50.0 %
A)4.76 %
B)5.00 %
C)5.26 %
D)50.0 %

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which is not a colligative property?
A)Boiling point
B)Freezing point
C)Density
D)Osmotic pressure
A)Boiling point
B)Freezing point
C)Density
D)Osmotic pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which salt is least soluble in water?
A)Sodium chloride
B)Iron(II)sulfide
C)Copper(II)bromide
D)Ammonium sulfide
A)Sodium chloride
B)Iron(II)sulfide
C)Copper(II)bromide
D)Ammonium sulfide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which one molar solution would have the highest boiling point?
A)KCl
B)BaCl2
C)AlCl3
D)CH3OH
A)KCl
B)BaCl2
C)AlCl3
D)CH3OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
37
What mass of NaOH is needed to produce 400.g of a solution that is 12.0 % by mass?
A)4.80 g
B)48.0 g
C)352 g
D)4800 g
A)4.80 g
B)48.0 g
C)352 g
D)4800 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which anion will not precipitate silver ion?
A)Chloride
B)Nitrate
C)Bromide
D)Carbonate
A)Chloride
B)Nitrate
C)Bromide
D)Carbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
39
The freezing point of pure water is 273 K.As a solute dissolves in water,the freezing point of the solution will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
40
Liquids which are capable of mixing and forming a solution are
A)unsaturated.
B)dilute.
C)miscible.
D)immiscible.
A)unsaturated.
B)dilute.
C)miscible.
D)immiscible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the molarity of a solution in which 0.300 moles of glucose is dissolved in 500.mL of solution?
A)1.67 M
B)0.600 M
C)0.0006 M
D)150.M
A)1.67 M
B)0.600 M
C)0.0006 M
D)150.M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
42
How many mL of a 4.50 (m/v)% solution would contain 23.0 g of solute?
A)19.6 mL
B)511 mL
C)1.04 mL
mL
D)10.35 mL
A)19.6 mL
B)511 mL
C)1.04
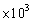 mL
mLD)10.35 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
43
How many parts per million of solute is present in a solution that is 0.300 % by mass?
A)0.300 ppm
B)30.0 ppm
C)300.ppm
D)3000 ppm
A)0.300 ppm
B)30.0 ppm
C)300.ppm
D)3000 ppm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
44
A solution is prepared by mixing 50.0 mL of ethanol with 300.0 mL of water.What is the volume percent of ethanol in this solution? Assume volumes are additive.
A)14.3 %
B)16.7 %
C)25.0 %
D)50.0 %
A)14.3 %
B)16.7 %
C)25.0 %
D)50.0 %

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
45
What mass of glucose is dissolved in 1000.g of a solution that is 5.00 % by mass?
A)5.00 g
B)50.0 g
C)5.00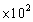 g
g
D)5.00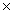 103 g
103 g
A)5.00 g
B)50.0 g
C)5.00
 g
gD)5.00
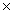 103 g
103 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the molarity of a solution in which 0.800 moles of sodium hydroxide is dissolved in 1500.mL of solution?
A)0.0533 M
B)53.3 M
C)5.33 M
D)0.533 M
A)0.0533 M
B)53.3 M
C)5.33 M
D)0.533 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the molarity of the resulting solution when 300.mL of a 0.600 M aqueous solutionis diluted by the addition of 400.mL of water?
A)0.257 M
B)0.450 M
C)0.800 M
D)1.40 M
A)0.257 M
B)0.450 M
C)0.800 M
D)1.40 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the molarity of the resulting solution when 20.0 mL of a 2.00 M aqueous solution is diluted by the addition of 30.0 mL of water?
A)5.00 M
B)3.00 M
C)1.33 M
D)0.800 M
A)5.00 M
B)3.00 M
C)1.33 M
D)0.800 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the molarity of a solution in which 55.49 g of calcium chloride is dissolved in enough water to make 500.mL of solution?
A)0.500 M
B)1.00 M
C)2.00 M
D)4.00 M
A)0.500 M
B)1.00 M
C)2.00 M
D)4.00 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the molality of a solution prepared by dissolving 40.0 g of glucose (C6H12O6)in 4.00 kg of water?
A)0.0555 m
B)10.0 m
C)0.0100 m
D)0.222 m
A)0.0555 m
B)10.0 m
C)0.0100 m
D)0.222 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the molarity of the resulting solution when 300.mL of a 0.400 M solution is diluted to 800.mL?
A)0.109 M
B)0.150 M
C)1.07 M
D)1.47 M
A)0.109 M
B)0.150 M
C)1.07 M
D)1.47 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the molarity of a solution in which 4.00 moles of potassium chloride is dissolved in 3.00 L of solution?
A)1.33 M
B)6.00 M
C)2.67 M
D)4.00 M
A)1.33 M
B)6.00 M
C)2.67 M
D)4.00 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
53
What mass of KCl is dissolved in 250.mL of a solution that is 4.00 % by mass/volume?
A)4.00 g
B)10.0 g
C)40.0 g
D)100.g
A)4.00 g
B)10.0 g
C)40.0 g
D)100.g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
54
How many moles of solute are dissolved in 400.mL of a 0.200 M solution?
A)0.0800 moles
B)0.800 moles
C)8.00 moles
D)80.0 moles
A)0.0800 moles
B)0.800 moles
C)8.00 moles
D)80.0 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
55
A 20.0 % solution of KCl has a mass of 400.g.What mass of KCl is contained in this solution?
A)20.0 g
B)80.0 g
C)320.g
D)400.g
A)20.0 g
B)80.0 g
C)320.g
D)400.g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
56
A solution is prepared by dissolving 0.400 g of solute in 1000.g of water.How many parts per million of solute are in this solution?
A)0.400 ppm
B)4.00 ppm
C)400.ppm
D)4000 ppm
A)0.400 ppm
B)4.00 ppm
C)400.ppm
D)4000 ppm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
57
What is the molality of a solution in which 0.3 moles of solute is dissolved in 1500.g of solvent?
A)0.0002 m
B)0.002 m
C)0.02 m
D)0.2 m
A)0.0002 m
B)0.002 m
C)0.02 m
D)0.2 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
58
A solution is prepared by mixing 20.0 mL of methanol with enough water to produce 400.0 mL of solution.What is the volume percent of methanol in this solution?
A)0.500 %
B)4.76 %
C)5.00 %
D)5.26 %
A)0.500 %
B)4.76 %
C)5.00 %
D)5.26 %

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the molarity of the resulting solution when 400.mL of a 0.400 M solution is diluted to 800.mL?
A)1.20 M
B)0.800 M
C)0.200 M
D)0.133 M
A)1.20 M
B)0.800 M
C)0.200 M
D)0.133 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the molarity of a solution in which 1.20 moles of solute is dissolved in 3.00 L of solution?
A)0.400 M
B)15.0 M
C)3.60 M
D)360.M
A)0.400 M
B)15.0 M
C)3.60 M
D)360.M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
61
In order to decrease the freezing point of 500.g of water to 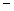 1.0°C,how many grams of ethylene glycol (C2H6O2)must be added? (Kf = 1.86
1.0°C,how many grams of ethylene glycol (C2H6O2)must be added? (Kf = 1.86 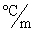 )
)
A)5.37 g
B)1.08 g
C)16.7 g
D)66.7 g
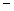 1.0°C,how many grams of ethylene glycol (C2H6O2)must be added? (Kf = 1.86
1.0°C,how many grams of ethylene glycol (C2H6O2)must be added? (Kf = 1.86 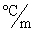 )
)A)5.37 g
B)1.08 g
C)16.7 g
D)66.7 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the boiling point of a 4.00 m aqueous solution of a nonvolatile nonelectrolyte? (The boiling point elevation constant for water is 0.512° C/m)
A)100.° C
B)102° C
C)98.0° C
D)2.05° C
A)100.° C
B)102° C
C)98.0° C
D)2.05° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
63
How many moles of KCl are dissolved in 1.20 L of a 0.400 M KCl solution?
A)0.333 moles
B)0.400 moles
C)0.480 moles
D)3.00 moles
A)0.333 moles
B)0.400 moles
C)0.480 moles
D)3.00 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
64
How would you prepare 150.0 mL of a 0.200 M aqueous solution of potassium chloride from a 0.500 M aqueous solution of potassium chloride?
A)Add 60.0 mL of water to the 0.500 M solution.
B)Take 60.0 mL of the 0.500 M solution and add enough water to complete to 150.0 mL of solution.
C)Add 90.0 mL of water to the 0.500 M solution.
D)Take 90.0 mL of the 0.500 M solution and add enough water to complete to 150.0 mL of solution.
A)Add 60.0 mL of water to the 0.500 M solution.
B)Take 60.0 mL of the 0.500 M solution and add enough water to complete to 150.0 mL of solution.
C)Add 90.0 mL of water to the 0.500 M solution.
D)Take 90.0 mL of the 0.500 M solution and add enough water to complete to 150.0 mL of solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
65
How many grams of Zn(s)will react completely with 100.0 mL of 0.0525 M HCl according to the following reaction? Zn(s)+ 2 HCl(aq)  ZnCl2(aq)+ H2(g)
ZnCl2(aq)+ H2(g)
A)0.172 g
B)5.25 g
C)0.343 g
D)0.687 g
 ZnCl2(aq)+ H2(g)
ZnCl2(aq)+ H2(g)A)0.172 g
B)5.25 g
C)0.343 g
D)0.687 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
66
When compared to pure water,aqueous solutions always have
A)higher boiling point and higher freezing point.
B)lower boiling point and lower freezing point.
C)higher boiling point and lower freezing point.
D)lower boiling point and higher freezing point.
A)higher boiling point and higher freezing point.
B)lower boiling point and lower freezing point.
C)higher boiling point and lower freezing point.
D)lower boiling point and higher freezing point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the boiling point of a 6.00 m aqueous solution of a nonvolatile nonelectrolyte? (The boiling point elevation constant for water is 0.512° C/m)
A)100.° C
B)103.° C
C)3.07° C
D)96.9° C
A)100.° C
B)103.° C
C)3.07° C
D)96.9° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
68
How many moles of solute are present in 300.mL of a 0.240 M solution?
A)0.0720 moles
B)0.240 moles
C)0.800 moles
D)1.25 moles
A)0.0720 moles
B)0.240 moles
C)0.800 moles
D)1.25 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the freezing point of a 0.400 m aqueous solution of a nonvolatile nonelectrolyte? ( The freezing point depression constant for water is 1.860 C/m)
A)0.744° C
B)0.00° C
C)-7.44° C
D)-0.744° C
A)0.744° C
B)0.00° C
C)-7.44° C
D)-0.744° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
70
How many liters of Cl2 gas at a pressure of 0.950 atm and a temperature of 298K will be collected from the reaction of 25.0 mL of a 0.100 M aqueous solution of KMnO4 and an excess of HCl? 2 KMnO4(aq)+ 16 HCl(aq)  2 MnCl2(aq)+ 5 Cl2(g)+ 8 H2O(l)+ 2 KCl(aq)
2 MnCl2(aq)+ 5 Cl2(g)+ 8 H2O(l)+ 2 KCl(aq)
A)0.321 L
B)0.0644 L
C)0.145 L
D)0.161 L
 2 MnCl2(aq)+ 5 Cl2(g)+ 8 H2O(l)+ 2 KCl(aq)
2 MnCl2(aq)+ 5 Cl2(g)+ 8 H2O(l)+ 2 KCl(aq)A)0.321 L
B)0.0644 L
C)0.145 L
D)0.161 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
71
What mass of magnesium sulfate is dissolved in 400.mL of a 0.800 M solution of magnesium sulfate?
A)0.320 g
B)2.00 g
C)38.5 g
D)241 g
A)0.320 g
B)2.00 g
C)38.5 g
D)241 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
72
What mass of silver nitrate is dissolved in 40.0 mL of a 0.400 M solution of silver nitrate?
A)0.272 g
B)2.72 g
C)27.2 g
D)272 g
A)0.272 g
B)2.72 g
C)27.2 g
D)272 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
73
What is the molarity of a solution which contains 9.12 g of hydrogen chloride in 200.mL of solution?
A)0.250 M
B)0.500 M
C)1.00 M
D)1.25 M
A)0.250 M
B)0.500 M
C)1.00 M
D)1.25 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
74
What mass of calcium chloride is dissolved in 100.mL of a 1.20 M solution of calcium chloride?
A)0.120 g
B)1.20 g
C)13.3 g
D)133 g
A)0.120 g
B)1.20 g
C)13.3 g
D)133 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
75
What mass of water must be added to 30.5 g of KNO3 to prepare a 45.0% by mass solution?
A)13.7 g
B)44.2 g
C)37.3 g
D)67.8 g
A)13.7 g
B)44.2 g
C)37.3 g
D)67.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
76
What is the boiling point of a 3.40 m aqueous solution of a nonvolatile nonelectrolyte? (The boiling point elevation constant for water is 0.512° C/m)
A)1.74° C
B)98.3° C
C)100.° C
D)102° C
A)1.74° C
B)98.3° C
C)100.° C
D)102° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the freezing point of a 1.40 m aqueous solution of a nonvolatile nonelectrolyte? ( The freezing point depression constant for water is 1.860 C/m)
A)-2.600 C
B)0.000 C
C)2.600 C
D)102.600 C
A)-2.600 C
B)0.000 C
C)2.600 C
D)102.600 C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
78
What is the freezing point of a 3.00 m aqueous solution of a nonvolatile nonelectrolyte? (The freezing point depression for water is 1.86° C/m)
A)-3.00° C
B)-5.58° C
C)3.00° C
D)5.58° C
A)-3.00° C
B)-5.58° C
C)3.00° C
D)5.58° C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the molarity of a solution which contains 85.00 g of sodium nitrate dissolved in 2.00 L of solution?
A)0.500 M
B)1.00 M
C)2.00 M
D)4.00 M
A)0.500 M
B)1.00 M
C)2.00 M
D)4.00 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
80
How many moles of KF are dissolved in 1400.mL of a 3.00 M KF solution?
A)0.467 moles
B)2.14 moles
C)3.00 moles
D)4.20 moles
A)0.467 moles
B)2.14 moles
C)3.00 moles
D)4.20 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck








