Deck 15: Acids, Bases, and Salts
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/100
العب
ملء الشاشة (f)
Deck 15: Acids, Bases, and Salts
1
Arrhenius defined a base as a substance whose aqueous solution contains an excess of
A)sodium ion.
B)hydroxide ion.
C)chloride ion.
D)hydrogen ion.
A)sodium ion.
B)hydroxide ion.
C)chloride ion.
D)hydrogen ion.
hydroxide ion.
2
In the following reaction,  the compound CH3NH2 behaves as:
the compound CH3NH2 behaves as:
A)an acid.
B)a base.
C)a salt.
D)a conjugate acid.
 the compound CH3NH2 behaves as:
the compound CH3NH2 behaves as:A)an acid.
B)a base.
C)a salt.
D)a conjugate acid.
a base.
3
Which is a strong electrolyte?
A)water
B)acetic acid
C)ammonia
D)sodium chloride
A)water
B)acetic acid
C)ammonia
D)sodium chloride
acetic acid
4
Which is a strong electrolyte?
A)sulfuric acid
B)sulfurous acid
C)ammonia
D)acetic acid
A)sulfuric acid
B)sulfurous acid
C)ammonia
D)acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
5
The hydroxide ion is responsible for the properties of
A)acids
B)bases
C)salts
A)acids
B)bases
C)salts

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
6
Bronsted and Lowry defined a base as a(n)
A)electron donor.
B)electron acceptor.
C)proton donor.
D)proton acceptor.
A)electron donor.
B)electron acceptor.
C)proton donor.
D)proton acceptor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
7
An amphoteric substance is one that
A)may react as an acid or a conjugate acid
B)may react as a salt
C)may react as a base or a conjugate base
D)may react as an acid or a base
A)may react as an acid or a conjugate acid
B)may react as a salt
C)may react as a base or a conjugate base
D)may react as an acid or a base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
8
Acids react with carbonates to produce the gas
A)hydrogen.
B)carbon dioxide.
C)nitrogen.
D)oxygen.
A)hydrogen.
B)carbon dioxide.
C)nitrogen.
D)oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
9
Bronsted and Lowry defined an acid as a(n)
A)electron donor.
B)electron acceptor.
C)proton donor.
D)proton acceptor.
A)electron donor.
B)electron acceptor.
C)proton donor.
D)proton acceptor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
10
In the following reaction,  water behaves as:
water behaves as:
A)an acid.
B)a base.
C)a salt.
D)a conjugate base.
 water behaves as:
water behaves as:A)an acid.
B)a base.
C)a salt.
D)a conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
11
In an acidic solution the
A)concentration of hydronium ion is greater than that of hydroxide ion
B)concentration of hydroxide ion greater than that of hydronium ion
C)concentration of hydronium ion and hydroxide ion are equal
A)concentration of hydronium ion is greater than that of hydroxide ion
B)concentration of hydroxide ion greater than that of hydronium ion
C)concentration of hydronium ion and hydroxide ion are equal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
12
A hydrogen ion consists of a(n)
A)electron.
B)proton.
C)neutron.
D)proton and electron.
A)electron.
B)proton.
C)neutron.
D)proton and electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
13
The hydronium ion is responsible for the properties of
A)acids
B)bases
C)salts
A)acids
B)bases
C)salts

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
14
The concentration of an aqueous solution of iron(II)chloride is 0.0550 M.What is the molarity of each ion in this solution?
A)0.0550 M for both the iron(II)and chloride ions.
B)0.0550 M for the iron(II)ion and 0.110 M for the chloride ion.
C)0.110 M for the iron(II)ion and 0.0550 M for the chloride ion.
D)not enough information is given to determine each molarity.
A)0.0550 M for both the iron(II)and chloride ions.
B)0.0550 M for the iron(II)ion and 0.110 M for the chloride ion.
C)0.110 M for the iron(II)ion and 0.0550 M for the chloride ion.
D)not enough information is given to determine each molarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
15
Acids react with active metals to produce the gas
A)nitrogen monoxide.
B)carbon dioxide.
C)helium.
D)hydrogen.
A)nitrogen monoxide.
B)carbon dioxide.
C)helium.
D)hydrogen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
16
Arrhenius defined an acid as a substance whose aqueous solution contains an excess of
A)sodium ion.
B)hydroxide ion.
C)chloride ion.
D)hydrogen ion.
A)sodium ion.
B)hydroxide ion.
C)chloride ion.
D)hydrogen ion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which is the hydroxide ion?
A)H +1
B)H3O +1
C)OH -1
D)OH2 -1
A)H +1
B)H3O +1
C)OH -1
D)OH2 -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which is a strong electrolyte?
A)methanol,CH3OH
B)water
C)hydrosulfuric acid
D)hydrochloric acid
A)methanol,CH3OH
B)water
C)hydrosulfuric acid
D)hydrochloric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
19
In a basic solution the
A)concentration of hydronium ion is greater than that of hydroxide ion
B)concentration of hydroxide ion is greater than that of hydronium ion
C)concentration of hydronium ion and hydroxide ion are equal
A)concentration of hydronium ion is greater than that of hydroxide ion
B)concentration of hydroxide ion is greater than that of hydronium ion
C)concentration of hydronium ion and hydroxide ion are equal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which is a strong electrolyte?
A)sugar
B)hypochlorous acid
C)sodium hydroxide
D)hydrosulfuric acid
A)sugar
B)hypochlorous acid
C)sodium hydroxide
D)hydrosulfuric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which pH is most acidic?
A)3
B)7
C)9
D)14
A)3
B)7
C)9
D)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which is the pH of a solution in which the concentration of hydroxide ion is greater than the concentration of hydrogen ion?
A)7
B)2
C)6
D)13
A)7
B)2
C)6
D)13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
23
In a 0.125 M aqueous solution of potassium phosphate,what is the molarity of each ion?
A)0.125 M for the potassium ion and 0.250 M for the phosphate ion.
B)0.125 M for the potassium ion and 0.375 M for the phosphate ion.
C)0.375 M for the potassium ion and 0.125 M for the phosphate ion.
D)0.250 M for the potassium ion and 0.125 M for the phosphate ion.
A)0.125 M for the potassium ion and 0.250 M for the phosphate ion.
B)0.125 M for the potassium ion and 0.375 M for the phosphate ion.
C)0.375 M for the potassium ion and 0.125 M for the phosphate ion.
D)0.250 M for the potassium ion and 0.125 M for the phosphate ion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which will ionize when placed in water?
A)KOH
B)HCl
C)KCl
D)LiCl
A)KOH
B)HCl
C)KCl
D)LiCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
25
A solution of a strong acid has a pH of 3.The concentration of hydronium ions in this solution is
A)0.001 M
B)3 M
C)0.3 M.
D)0.003 M
A)0.001 M
B)3 M
C)0.3 M.
D)0.003 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which acid and base will combine to form sodium sulfite?
A)NaOH and H2SO3
B)NaOH and H2SO4
C)Na(OH)2 and H2SO3
D)Na(OH)2 and H2SO4
A)NaOH and H2SO3
B)NaOH and H2SO4
C)Na(OH)2 and H2SO3
D)Na(OH)2 and H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which will dissociate when placed in water?
A)CH3OH
B)HCl
C)NaCl
D)HClO4
A)CH3OH
B)HCl
C)NaCl
D)HClO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which is the pH of a solution in which the concentration of hydrogen ion is equal to the concentration of hydroxide ion?
A)7
B)9
C)12
D)4
A)7
B)9
C)12
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
29
Combining potassium hydroxide and sulfuric acid will produce water and
A)potassium sulfide.
B)potassium sulfate.
C)potassium sulfite.
D)potassium hypersulfate.
A)potassium sulfide.
B)potassium sulfate.
C)potassium sulfite.
D)potassium hypersulfate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which is a strong base?
A)CuOH
B)HCl
C)KOH
D)CH3OH
A)CuOH
B)HCl
C)KOH
D)CH3OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which is a strong acid?
A)HNO3
B)HNO2
C)HClO
D)HClO2
A)HNO3
B)HNO2
C)HClO
D)HClO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which pH is most alkaline?
A)1
B)5
C)7
D)12
A)1
B)5
C)7
D)12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which pH is neutral?
A)2
B)4
C)5
D)7
A)2
B)4
C)5
D)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which acid and base will combine to form calcium sulfate?
A)CaOH and H2SO3
B)CaOH and H2SO4
C)Ca(OH)2 and H2SO3
D)Ca(OH)2 and H2SO4
A)CaOH and H2SO3
B)CaOH and H2SO4
C)Ca(OH)2 and H2SO3
D)Ca(OH)2 and H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which is a strong base?
A)NH4OH
B)Ca(OH)2
C)HNO2
D)HNO3
A)NH4OH
B)Ca(OH)2
C)HNO2
D)HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
36
How many times more concentrated is the hydrogen ion concentration in a solution with a pH of 3 than that with a pH of 5?
A)2 times
B)10 times
C)100 times
D)1000 times
A)2 times
B)10 times
C)100 times
D)1000 times

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which type of compound is not an electrolyte?
A)Alcohol
B)Acid
C)Base
D)Salt
A)Alcohol
B)Acid
C)Base
D)Salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which is the pH of a solution in which the concentration of hydrogen ion is greater than the concentration of hydroxide ion?
A)12
B)9
C)7
D)3
A)12
B)9
C)7
D)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
39
Combining sodium hydroxide and hydrochloric acid will produce water and
A)sodium chloride.
B)sodium chlorite.
C)sodium chlorate.
D)sodium hypochlorite.
A)sodium chloride.
B)sodium chlorite.
C)sodium chlorate.
D)sodium hypochlorite.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which is a strong acid?
A)HClO3
B)HClO2
C)H2SO3
D)H2SO4
A)HClO3
B)HClO2
C)H2SO3
D)H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the concentration of calcium ion in a 2.0 M solution of calcium chloride?
A)1.0 M
B)2.0 M
C)3.0 M
D)4.0 M
A)1.0 M
B)2.0 M
C)3.0 M
D)4.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the boiling point of a 2.0 m aqueous solution of sodium chloride? (The boiling point elevation constant for water is 0.512 °C/m. )
A)101.02 °C
B)1.02 °C
C)2.05 °C
D)102.05 °C
A)101.02 °C
B)1.02 °C
C)2.05 °C
D)102.05 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which is a conjugate acid base pair in the following equation? H3PO4 + H2O  H3O +1 + H2PO4 -1
H3O +1 + H2PO4 -1
A)H3PO4 and H2O
B)H3O +1 and H2PO4 -1
C)H2O and H3O +1
D)H3PO4 and H3O +1
 H3O +1 + H2PO4 -1
H3O +1 + H2PO4 -1A)H3PO4 and H2O
B)H3O +1 and H2PO4 -1
C)H2O and H3O +1
D)H3PO4 and H3O +1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the conjugate acid of OH -1?
A)O-2
B)H +1
C)O2H
D)HOH
A)O-2
B)H +1
C)O2H
D)HOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the concentration of chloride ion in a 2.0 M solution of calcium chloride?
A)1.0 M
B)2.0 M
C)3.0 M
D)4.0 M
A)1.0 M
B)2.0 M
C)3.0 M
D)4.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which are the two Bronsted-Lowry bases in the following equation? NH3 + H2O  NH2 -1 + H3O +1
NH2 -1 + H3O +1
A)H2O and NH2 -1
B)NH3 and H2O
C)H3O +1 and NH3
D)NH2 -1 and H3O +1
 NH2 -1 + H3O +1
NH2 -1 + H3O +1A)H2O and NH2 -1
B)NH3 and H2O
C)H3O +1 and NH3
D)NH2 -1 and H3O +1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the conjugate acid of NH3?
A)N-3
B)NH4 +1
C)NH2 -1
D)NH -2
A)N-3
B)NH4 +1
C)NH2 -1
D)NH -2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which is a conjugate acid base pair in the following equation? H2SO4 + H2O  HSO4 -1 + H3O +1
HSO4 -1 + H3O +1
A)H2SO4 and HSO4 -1
B)H2SO4 and H2O
C)HSO4 -1 and H3O +1
D)H3O +1 and H2SO4
 HSO4 -1 + H3O +1
HSO4 -1 + H3O +1A)H2SO4 and HSO4 -1
B)H2SO4 and H2O
C)HSO4 -1 and H3O +1
D)H3O +1 and H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the net ionic equation when hydrobromic acid reacts with potassium hydroxide?
A)H +1 + OH -1 H2O
B)H +1 + Br--1 HBr
C)K +1 + OH -1 KOH
D)K +1 + H-1 KH
A)H +1 + OH -1 H2O
B)H +1 + Br--1 HBr
C)K +1 + OH -1 KOH
D)K +1 + H-1 KH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
50
What are the spectator ions when hydrochloric acid reacts with sodium hydroxide?
A)Na +1 and H +1
B)Cl -1 and OH -1
C)H +1 and OH -1
D)Na +1 and Cl -1
A)Na +1 and H +1
B)Cl -1 and OH -1
C)H +1 and OH -1
D)Na +1 and Cl -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
51
The pH of a solution can be calculated from the expression
A)pH = - log [OH -1]
B)pH = log [OH -1]
C)pH = - log [H +1]
D)pH = log [H +1]
A)pH = - log [OH -1]
B)pH = log [OH -1]
C)pH = - log [H +1]
D)pH = log [H +1]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which is a conjugate acid base pair in the following equation? HS -1 + H2O  S -2 + H3O +1
S -2 + H3O +1
A)HS -1 and H3O +1
B)HS -1 and S -2
C)H3O +1 and S -2
D)HS -1 and H2O
 S -2 + H3O +1
S -2 + H3O +1A)HS -1 and H3O +1
B)HS -1 and S -2
C)H3O +1 and S -2
D)HS -1 and H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which are the two Bronsted-Lowry acids in the following equation? NH3 + H2O  NH4 +1 + OH -1
NH4 +1 + OH -1
A)NH3 and H2O
B)NH3 and NH4 +1
C)NH3 and OH -1
D)NH4 +1 and H2O
 NH4 +1 + OH -1
NH4 +1 + OH -1A)NH3 and H2O
B)NH3 and NH4 +1
C)NH3 and OH -1
D)NH4 +1 and H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
54
What is the conjugate base of NH3?
A)NH2 -1
B)NH -2
C)NH4 +1
D)H +1
A)NH2 -1
B)NH -2
C)NH4 +1
D)H +1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which are the two Bronsted-Lowry acids in the following equation? H2S + H2O  HS -1 + H3O +1
HS -1 + H3O +1
A)HS -1 and H2O
B)H2S and HS -1
C)H2S and H3O +1
D)H3O +1 and H2O
 HS -1 + H3O +1
HS -1 + H3O +1A)HS -1 and H2O
B)H2S and HS -1
C)H2S and H3O +1
D)H3O +1 and H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which is a conjugate acid base pair in the following equation? HF + H2O  H3O +1 + F -1
H3O +1 + F -1
A)HF and H3O +1
B)HF and H2O
C)H3O +1 and F -1
D)HF and F -1
 H3O +1 + F -1
H3O +1 + F -1A)HF and H3O +1
B)HF and H2O
C)H3O +1 and F -1
D)HF and F -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
57
A solution of a strong base has a pH of 11.The concentration of hydronium ions in this solution is
A)11 M
B)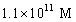
C)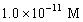
D)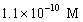
A)11 M
B)
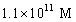
C)
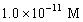
D)
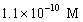

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the conjugate base of HS -1?
A)H2S
B)S -2
C)H +1
D)OH -1
A)H2S
B)S -2
C)H +1
D)OH -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which are the two Bronsted-Lowry bases in the following equation? HNO2 + H2O  H3O +1 + NO2 -1
H3O +1 + NO2 -1
A)H3O +1 and HNO2
B)H2O and NO2 -1
C)HNO2 and H2O
D)H3O +1 and NO2 -1
 H3O +1 + NO2 -1
H3O +1 + NO2 -1A)H3O +1 and HNO2
B)H2O and NO2 -1
C)HNO2 and H2O
D)H3O +1 and NO2 -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which type of mixture displays Brownian motion and the Tyndall effect?
A)Solution
B)Colloidal dispersion
C)Suspension
A)Solution
B)Colloidal dispersion
C)Suspension

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
61
What is the pH of a 0.01 M solution of hydrochloric acid?
A)0.01
B)1
C)2
D)-2
A)0.01
B)1
C)2
D)-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the freezing point of a 1.0 m aqueous solution of CaCl2? (The freezing point depression constant for water is 1.86 °C/m. )
A)-1.86 °C
B)1.86 °C
C)-3.72 °C
D)-5.58 °C
A)-1.86 °C
B)1.86 °C
C)-3.72 °C
D)-5.58 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the concentration of an HBr solution if 12.0 mL of the solution is neutralized by 15.0 mL of a 0.25 M KOH solution?
A)0.75 M
B)0.31 M
C)0.40 M
D)0.20 M
A)0.75 M
B)0.31 M
C)0.40 M
D)0.20 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the concentration of an HCl solution if 25.0 mL of the solution is neutralizedcompletely by 40.0 mL of a 0.30 M KOH solution?
A)0.19 M
B)300 M
C)46.9 M
D)0.48 M
A)0.19 M
B)300 M
C)46.9 M
D)0.48 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
65
An aqueous solution is 18.7% by mass Na3PO4.What is the boiling point of this solution? The Kb for water is 0.512°C/m.Consider if the solute is an electrolyte or a nonelectrolyte.
A)102.89°C
B)100.72°C
C)101.44°C
D)100.00 °C
A)102.89°C
B)100.72°C
C)101.44°C
D)100.00 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the pH of a solution whose H +1 concentration is 4.0 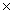 10 -9?
10 -9?
A)4.0
B)8.4
C)3.6
D)9.0
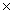 10 -9?
10 -9?A)4.0
B)8.4
C)3.6
D)9.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the pH of a 1.0 M solution of nitric acid?
A)0
B)1
C)-1
D)4
A)0
B)1
C)-1
D)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the pH of a 0.034 M hydrochloric acid solution?
A)-2
B)3.4
C)-1.5
D)1.5
A)-2
B)3.4
C)-1.5
D)1.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the concentration of a H2SO4 solution if 10.0 mL of the solution is neutralized by 7.8 mL of a 0.20 M NaOH solution?
A)0.0026 M
B)0.078 M
C)0.156 M
D)0.31 M
A)0.0026 M
B)0.078 M
C)0.156 M
D)0.31 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the concentration of a HNO3 solution if 10.0 mL of the solution is neutralized by 3.6 mL of a 0.20 M NaOH solution?
A)0.072 M
B)53.6 M
C)0.56 M
D)5.6 M
A)0.072 M
B)53.6 M
C)0.56 M
D)5.6 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
71
What is the boiling point of a 0.50 m aqueous solution of Al(NO3)3? (The boiling point elevation constant for water is 0.512 °C/m. )
A)100.51 °C
B)101.02 °C
C)102.05 °C
D)106.60 °C
A)100.51 °C
B)101.02 °C
C)102.05 °C
D)106.60 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the concentration of a H2SO4 solution if 15.0 mL of the solution is neutralized by 3.60 mL of a 0.65 M Ca(OH)2 solution?
A)0.078 M
B)0.16 M
C)0.31 M
D)2.7 M
A)0.078 M
B)0.16 M
C)0.31 M
D)2.7 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
73
An aqueous solution is 12.5% in Na2CO3 and has a density of 1.19 g/mL.What is the molarity of this solution?
A)0.117 M
B)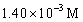
C)14.9 M
D)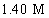
A)0.117 M
B)
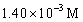
C)14.9 M
D)
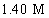

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the concentration of a HCl solution if 20.0 mL of the solution is neutralized by 15.0 mL of a 0.10 M Ca(OH)2 solution?
A)0.075 M
B)0.038 M
C)0.15 M
D)0.13 M
A)0.075 M
B)0.038 M
C)0.15 M
D)0.13 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the pH of a solution whose H3O +1 concentration is 1 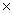 10 -9?
10 -9?
A)1
B)9
C)-9
D)-1
 10 -9?
10 -9?A)1
B)9
C)-9
D)-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
76
What mass of ethanol,C2H5OH,should be added to 200.0 g of water in order to increase its boiling point to 102.5°C? The Kb for water is 0.512°C/m.Consider if the solute is an electrolyte or a nonelectrolyte.
A)4.9 g
B)0.98 g
C)45 g
D)90.g
A)4.9 g
B)0.98 g
C)45 g
D)90.g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
77
What mass of sodium chloride should be added to 500.0 g of water in order to decrease its freezing point to -1.0 °C? The Kf for water is 1.86°C/m.Consider if the solute is an electrolyte or a nonelectrolyte.
A)0.269 g
B)134 g
C)15.7 g
D)7.85 g
A)0.269 g
B)134 g
C)15.7 g
D)7.85 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
78
A solution is prepared by adding 35.5 mL of ethylene glycol,C2H6O2,to 250.0 g of water.What is the freezing point of this solution? The Kf for water is 1.86°C/m and the density of ethylene glycol is 1.1135 g/mL.Consider if the solute is an electrolyte or a nonelectrolyte.
A)−0.29°C
B)−4.74°C
C)−0.01°C
D)0.00°C
A)−0.29°C
B)−4.74°C
C)−0.01°C
D)0.00°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the pH of a 0.020 M nitric acid solution?
A)0.020
B)-2.0
C)1.7
D)4.0
A)0.020
B)-2.0
C)1.7
D)4.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the freezing point of a 3.0 m aqueous solution of NaCl? (The freezing point depression constant for water is 1.86 °C/m. )
A)5.6 °C
B)-5.6 °C
C)11 °C
D)-11 °C
A)5.6 °C
B)-5.6 °C
C)11 °C
D)-11 °C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck








