Deck 28: Atomic Physics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/73
العب
ملء الشاشة (f)
Deck 28: Atomic Physics
1
The Lyman series of hydrogen corresponds to electron transitions from higher levels to n = 1.What is the longest wavelength in that series? (R = 1.097 × 107 m−1 and 1 nm = 10−9 m)
A)91.4 nm
B)122 nm
C)273 nm
D)456 nm
A)91.4 nm
B)122 nm
C)273 nm
D)456 nm
122 nm
2
The ionization energy of the hydrogen atom is 13.6 eV.What is the energy of the n = 7 state?
A)11.7 eV
B)-11.7 eV
C)0.28 eV
D)-0.28 eV
E)1.94 eV
A)11.7 eV
B)-11.7 eV
C)0.28 eV
D)-0.28 eV
E)1.94 eV
-0.28 eV
3
The Lyman series of hydrogen is made up of those transitions made from higher levels to n = 1.If the fifth line in this series has a wavelength of 94 nm,what is the wavelength of the sixth line?
A)106 nm
B)93 nm
C)117 nm
D)102 nm
E)124 nm
A)106 nm
B)93 nm
C)117 nm
D)102 nm
E)124 nm
93 nm
4
When a wire carries high current causing it to glow,it will emit which type of spectrum?
A)line emission
B)line absorption
C)continuous
D)monochromatic
A)line emission
B)line absorption
C)continuous
D)monochromatic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
5
The Paschen series of hydrogen corresponds to electron transitions from higher levels to n = 3.What is the shortest wavelength in that series? (R = 1.097 × 107 m−1 and 1 nm = 10−9 m)
A)1875 nm
B)820 nm
C)1580 nm
D)656 nm
E)1094 nm
A)1875 nm
B)820 nm
C)1580 nm
D)656 nm
E)1094 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
6
Of the various wavelengths emitted from a hydrogen gas discharge tube,those associated with transitions from higher levels down to the n = 2 level produce which of the following?
A)infrared
B)visible
C)mixture of visible and ultraviolet
D)ultraviolet
A)infrared
B)visible
C)mixture of visible and ultraviolet
D)ultraviolet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
7
The ionization energy for the hydrogen atom is 13.6 eV.What is the energy of a photon that is emitted as a hydrogen atom makes a transition between the n = 6 and n = 4 states?
A)1.13 eV
B)0.47 eV
C)0.09 eV
D)3.40 eV
E)6.80 eV
A)1.13 eV
B)0.47 eV
C)0.09 eV
D)3.40 eV
E)6.80 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the wavelength of the line in the Balmer series of hydrogen that is comprised of transitions from the n = 5 to the n = 2 level? (R = 1.097 × 107 m−1 and 1 nm = 10−9 m)
A)304 nm
B)434 nm
C)476 nm
D)95 nm
E)333 nm
A)304 nm
B)434 nm
C)476 nm
D)95 nm
E)333 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the wavelength of the line in the Paschen series of hydrogen that is comprised of transitions from the n = 5 to the n = 3 levels? (R = 1.097 × 107 m−1 and 1 nm = 10−9 m)
A)1406 nm
B)1282 nm
C)684 nm
D)750 nm
E)365 nm
A)1406 nm
B)1282 nm
C)684 nm
D)750 nm
E)365 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
10
When a high voltage is applied to a low-pressure gas causing it to glow,it will emit which type of spectrum?
A)line emission
B)line absorption
C)continuous
D)monochromatic
A)line emission
B)line absorption
C)continuous
D)monochromatic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
11
The four visible colors emitted by hydrogen atoms are produced by electrons
A)that start in the ground state.
B)that end up in the ground state.
C)that start in the level with n = 2.
D)that end up in the level with n = 2.
A)that start in the ground state.
B)that end up in the ground state.
C)that start in the level with n = 2.
D)that end up in the level with n = 2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
12
The ionization energy of the hydrogen atom is 13.6 eV.What is the wavelength of a photon having this much energy? (h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.6 × 10−19 J,and 1 nm = 10−9 m)
A)91.4 nm
B)136 nm
C)273 nm
D)360 nm
A)91.4 nm
B)136 nm
C)273 nm
D)360 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
13
If the radius of the electron orbit in the n = 1 level of the hydrogen atoms is 0.052 9 nm,what is its radius for the n = 4 level? (Assume the Bohr model is valid).
A)0.212 nm
B)0.045 nm
C)0.716 nm
D)0.846 nm
E)0.920 nm
A)0.212 nm
B)0.045 nm
C)0.716 nm
D)0.846 nm
E)0.920 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
14
The visible lines from hydrogen are all members of the
A)Lyman series.
B)Balmer series.
C)Paschen series.
D)Brackett series.
A)Lyman series.
B)Balmer series.
C)Paschen series.
D)Brackett series.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
15
According to the Rutherford model of the atom,most of the volume of an atom
A)is empty space.
B)was occupied by the nucleus.
C)contained positive charges.
D)excluded electrons.
A)is empty space.
B)was occupied by the nucleus.
C)contained positive charges.
D)excluded electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
16
The ionization energy of the hydrogen atom is 13.6 eV.What is the energy of a photon emitted corresponding to a transition from the n = 5 to n = 3 state?
A)0.97 eV
B)1.81 eV
C)3.40 eV
D)6.80 eV
E)0.24 eV
A)0.97 eV
B)1.81 eV
C)3.40 eV
D)6.80 eV
E)0.24 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
17
In contrast to Thomson's model of the atom,Rutherford's model
A)had the positive charge spread uniformly through the atom.
B)had the positive charge concentrated in a small region.
C)was first to explain atoms emitting discrete frequencies.
D)eliminated radiation from accelerating charges.
A)had the positive charge spread uniformly through the atom.
B)had the positive charge concentrated in a small region.
C)was first to explain atoms emitting discrete frequencies.
D)eliminated radiation from accelerating charges.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
18
Of the various wavelengths emitted from a hydrogen gas discharge tube,those that are associated with transitions from higher levels down to the n = 1 level produce which of the following?
A)infrared
B)visible
C)mixture of infrared and visible
D)ultraviolet
A)infrared
B)visible
C)mixture of infrared and visible
D)ultraviolet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
19
An alpha particle is
A)a neutral helium atom.
B)any positively charged nucleus.
C)an x-ray.
D)None of the above.
A)a neutral helium atom.
B)any positively charged nucleus.
C)an x-ray.
D)None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
20
When a cool gas is placed between a glowing wire filament source and a diffraction grating,the resultant spectrum from the grating is which one of the following?
A)line emission
B)line absorption
C)continuous
D)monochromatic
A)line emission
B)line absorption
C)continuous
D)monochromatic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
21
In the hydrogen atom the potential energy is negative,but the absolute value of the potential energy
A)is equal to the kinetic energy of the electron.
B)is twice the kinetic energy of the electron.
C)is half the kinetic energy of the electron.
D)is equal to n2 times the kinetic energy of the electron.
A)is equal to the kinetic energy of the electron.
B)is twice the kinetic energy of the electron.
C)is half the kinetic energy of the electron.
D)is equal to n2 times the kinetic energy of the electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
22
The Bohr model of the hydrogen atom accounts for which quantum number?
A)principal
B)orbital
C)orbital magnetic
D)All of the above.
A)principal
B)orbital
C)orbital magnetic
D)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
23
In an analysis relating Bohr's theory to the de Broglie wavelength of electrons,when an electron moves from the n = 1 level to the n = 2 level,the circumference for its orbit becomes 4 times greater.This occurs because
A)there are four times as many wavelengths in the new orbit.
B)the wavelength of the electron becomes four times as long.
C)there are twice as many wavelengths,and each wavelength is twice as long.
D)the electron is moving four times as fast.
A)there are four times as many wavelengths in the new orbit.
B)the wavelength of the electron becomes four times as long.
C)there are twice as many wavelengths,and each wavelength is twice as long.
D)the electron is moving four times as fast.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
24
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number = 4,there will be how many permitted orbital magnetic quantum numbers allowed?
A)3
B)4
C)8
D)9
E)16
A)3
B)4
C)8
D)9
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
25
A muon behaves like an electron except that it has 207 times the mass of the electron.If a muon were bound to a proton,how would the energy levels in the Bohr model compare to those for a bound electron?
A)They would be the same.
B)They would be (207)2 times as much as those for the electron.
C)They would be 207 times as much as those for the electron.
D)They would be (1/207)times as much as those for the electron.
A)They would be the same.
B)They would be (207)2 times as much as those for the electron.
C)They would be 207 times as much as those for the electron.
D)They would be (1/207)times as much as those for the electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
26
A hydrogen atom in the ground state absorbs a 13.32-eV photon.To what level is the electron promoted? (The ionization energy of hydrogen is 13.6 eV).
A)n = 6
B)n = 10
C)n = 7
D)n = 5
E)n = 11
A)n = 6
B)n = 10
C)n = 7
D)n = 5
E)n = 11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
27
An energy of 13.6 eV is needed to remove an electron from the n = 3 state of a lithium atom.If a single photon accomplishes this task,what wavelength is needed? (h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.6 × 10−19 J,and 1 nm = 10−9 m)
A)146.3 nm
B)30.5 nm
C)13.8 nm
D)91.4 nm
E)234 nm
A)146.3 nm
B)30.5 nm
C)13.8 nm
D)91.4 nm
E)234 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
28
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number is 6,there will be how many different permitted orbital quantum number(s)?
A)5
B)12
C)6
D)36
E)13
A)5
B)12
C)6
D)36
E)13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
29
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number of the hydrogen atom is 7,there will be how many permitted orbital magnetic quantum numbers?
A)6
B)7
C)14
D)15
E)49
A)6
B)7
C)14
D)15
E)49

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the energy needed to change an He+ ion into an He++ ion? (The ionization energy of hydrogen is 13.6 eV).
A)13.6 eV
B)54.4 eV
C)92.9 eV
D)112.4 eV
A)13.6 eV
B)54.4 eV
C)92.9 eV
D)112.4 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
31
A photon is emitted from a hydrogen atom that undergoes a transition from n = 3 to n = 2.Calculate the energy and wavelength of the photon.(The ionization energy of hydrogen is 13.6 eV,and h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10−19 J,and 1 nm = 10−9 m)
A)1.9 eV,660 nm
B)1.9 eV,1050 nm
C)2.3 eV,550 nm
D)2.3 eV,660 nm
E)2.3 eV,1050 nm
A)1.9 eV,660 nm
B)1.9 eV,1050 nm
C)2.3 eV,550 nm
D)2.3 eV,660 nm
E)2.3 eV,1050 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
32
Consider the hydrogen atom,singly ionized helium atom,and the doubly ionized lithium atom.Arrange these atoms from highest energy ground state to lowest energy ground state.
A)H,He+,Li++
B)Li++,He+,H
C)H,Li++,He+
D)Since each of these atoms has only one electron,they all have the same energy ground state.
A)H,He+,Li++
B)Li++,He+,H
C)H,Li++,He+
D)Since each of these atoms has only one electron,they all have the same energy ground state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following transitions in hydrogen from an initial state (ni)to a final state (nf)results in the most energy emitted?
A)ni = 80,nf = 2
B)ni = 3,nf = 95
C)ni = 2,nf = 1
D)ni = 1,nf = 3
A)ni = 80,nf = 2
B)ni = 3,nf = 95
C)ni = 2,nf = 1
D)ni = 1,nf = 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
34
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 4,there will be how many permitted orbital quantum numbers?
A)2
B)4
C)8
D)16
E)32
A)2
B)4
C)8
D)16
E)32

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
35
The quantum mechanical model of the hydrogen atom requires that if the orbital magnetic quantum number is 3,there will be how many permitted spin magnetic quantum numbers?
A)2
B)3
C)4
D)6
E)7
A)2
B)3
C)4
D)6
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
36
Using quantum theories to study the hydrogen atom allows the prediction and experimental verification of many data about atoms such as He+ and Li2+.Some quantities that can successfully be predicted about He+ and Li2+ in this manner are:
A)the color of the light they will emit.
B)their mass.
C)their abundance in nature.
D)All of the above.
A)the color of the light they will emit.
B)their mass.
C)their abundance in nature.
D)All of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
37
The emission of a line from the Balmer series is followed almost immediately by the emission of a line from the Lyman series.This will be true for
A)only the first line of the Balmer series and the first line of the Lyman series.
B)all the lines of the Balmer series followed by only the first line of the Lyman series.
C)only the first line of the Balmer series followed by any of the lines of the Lyman series.
D)all the lines of the Balmer series followed by any of the lines of the Lyman series.
A)only the first line of the Balmer series and the first line of the Lyman series.
B)all the lines of the Balmer series followed by only the first line of the Lyman series.
C)only the first line of the Balmer series followed by any of the lines of the Lyman series.
D)all the lines of the Balmer series followed by any of the lines of the Lyman series.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
38
If a hydrogen atom,originally in its ground state of energy -13.6 eV,absorbs a photon of energy 15.9 eV,what is the resulting kinetic energy of the electron if the proton has negligible kinetic energy?
A)13.6 eV
B)-2.30 eV
C)2.30 eV
D)15.9 eV
E)Such a photon cannot be absorbed in this case.
A)13.6 eV
B)-2.30 eV
C)2.30 eV
D)15.9 eV
E)Such a photon cannot be absorbed in this case.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
39
The speed of the electron in the Bohr theory of hydrogen is
A)proportional to n.
B)proportional to n2.
C)inversely proportional to n.
D)inversely proportional to n2.
A)proportional to n.
B)proportional to n2.
C)inversely proportional to n.
D)inversely proportional to n2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
40
The Bohr theory does not predict that
A)hydrogen atoms will give off the lines from the Balmer series.
B)the ground state of hydrogen is spherically symmetric.
C)it requires 13.6 eV to ionize hydrogen.
D)the approximate radius of a hydrogen atom is 5.3 × 10−11 m.
A)hydrogen atoms will give off the lines from the Balmer series.
B)the ground state of hydrogen is spherically symmetric.
C)it requires 13.6 eV to ionize hydrogen.
D)the approximate radius of a hydrogen atom is 5.3 × 10−11 m.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
41
The quantum number that can have only two possible values is the
A)principal quantum number.
B)orbital quantum number.
C)orbital magnetic quantum number.
D)spin magnetic quantum number.
A)principal quantum number.
B)orbital quantum number.
C)orbital magnetic quantum number.
D)spin magnetic quantum number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the maximum number of electrons that can occupy a 3d subshell?
A)5
B)14
C)10
D)18
A)5
B)14
C)10
D)18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
43
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 5,there will be how many permitted orbital magnetic quantum numbers?
A)4
B)5
C)10
D)9
E)11
A)4
B)5
C)10
D)9
E)11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
44
For the n = 5 shell,what are the lowest values possible for 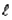
And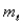
Respectively?
A)-5,-5
B)−4,-4
C)0,-4
D)0,0
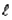
And
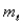
Respectively?
A)-5,-5
B)−4,-4
C)0,-4
D)0,0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
45
Imagine that an electron had a spin of 5/2 so that its spin quantum number,ms,could have the following six values: ms = +5/2,+3/2,+1/2,-1/2,-3/2,and -5/2.If this were true,the first element with a filled shell would be
A)He with 2 electrons.
B)Be with 4 electrons.
C)C with 6 electrons.
D)O with 8 electrons.
A)He with 2 electrons.
B)Be with 4 electrons.
C)C with 6 electrons.
D)O with 8 electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
46
In the n = 4 shell,how many distinct values of 
Are possible?
A)4
B)8
C)9
D)The correct value is not given.

Are possible?
A)4
B)8
C)9
D)The correct value is not given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many electrons are in chlorine's (atomic number 17)next to outer shell (n = 2)?
A)18
B)4
C)2
D)8
A)18
B)4
C)2
D)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
48
If the principal quantum number for hydrogen is 7,which one of the following is not a permitted orbital magnetic quantum number for that atom?
A)10
B)-2
C)0
D)5
E)3
A)10
B)-2
C)0
D)5
E)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
49
The quantity ________,where Ψ is the wave function,represents the probability per unit volume of finding an electron in that volume.
A)Ψ1/2
B)Ψ
C)Ψ3/2
D)Ψ2
A)Ψ1/2
B)Ψ
C)Ψ3/2
D)Ψ2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
50
How many electrons are in chromium's (atomic number 24)next to outer shell (n = 3)?
A)1
B)14
C)12
D)13
E)8
A)1
B)14
C)12
D)13
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
51
The ground state configuration for an element is 1s22s22p63s23p1.What is the atomic number of the element?
A)11
B)9
C)24
D)13
A)11
B)9
C)24
D)13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
52
The restriction that no more than one electron may occupy a given quantum state in an atom was first stated by which of the following scientists?
A)Bohr
B)de Broglie
C)Heisenberg
D)Pauli
A)Bohr
B)de Broglie
C)Heisenberg
D)Pauli

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
53
Magnesium has atomic number 12.In its ground state,how many electrons are in its n = 3 shell?
A)2
B)8
C)16
D)10
A)2
B)8
C)16
D)10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
54
The x-rays that occur when a high energy electron beam is incident on a metal target will show what type of spectrum?
A)continuous
B)line
C)continuous spectrum superimposed with a line spectrum
D)absorption
A)continuous
B)line
C)continuous spectrum superimposed with a line spectrum
D)absorption

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
55
The ground state electronic configuration for silicon is 1s22s22p63s23p2.Which of the following elements would be expected to be chemically similar to silicon?
A)boron with configuration 1s22s22p1
B)lithium with configuration 1s22s1
C)carbon with configuration 1s22s22p2
D)sodium with configuration 1s22s22p63s1
A)boron with configuration 1s22s22p1
B)lithium with configuration 1s22s1
C)carbon with configuration 1s22s22p2
D)sodium with configuration 1s22s22p63s1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
56
Aluminium has atomic number 13.In its ground state,how many electrons are in its n = 1 shell?
A)6
B)2
C)3
D)10
E)16
A)6
B)2
C)3
D)10
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
57
If the energy for the ground state level (n = 1)of hydrogen is −13.6 eV,which of the following gives an approximate value for the energy of an electron of the K shell (n = 1)of the element beryllium for which the atomic number = 4?
A)-122 eV
B)-40.8 eV
C)-218 eV
D)-54.4 eV
E)-40.8 eV
A)-122 eV
B)-40.8 eV
C)-218 eV
D)-54.4 eV
E)-40.8 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
58
The quantum mechanical model of the hydrogen atom suggests a visual picture of the electron as which of the following?
A)raisin in pudding
B)probability cloud
C)planetary orbiting body
D)light quantum
A)raisin in pudding
B)probability cloud
C)planetary orbiting body
D)light quantum

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
59
The ground state electronic configuration for sodium is 1s22s22p63s1.What is the orbital quantum number of the last (3s1)electron?
A)3
B)1
C)2
D)0
A)3
B)1
C)2
D)0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
60
In a plot of probability of finding the electron in the hydrogen ground state versus the distance from the nucleus,the maximum occurs
A)at a0,the first Bohr radius.
B)at slightly less than a0.
C)at slightly more than a0.
D)at 2 a0.
A)at a0,the first Bohr radius.
B)at slightly less than a0.
C)at slightly more than a0.
D)at 2 a0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following demonstrated the relation between the atomic number of a given element and the wavelength of the K-alpha x-ray photon emitted by that element?
A)Bohr
B)Compton
C)Moseley
D)Pauli
A)Bohr
B)Compton
C)Moseley
D)Pauli

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
62
The red light from a HeNe laboratory laser results from a transition in Ne.Determine the wavelength of the light given off if the energy difference between states is 1.08 eV.(h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10−19 J,and 1 nm = 10−9 m)
A)383 nm
B)1840 nm
C)1150 nm
D)1340 nm
E)203 nm
A)383 nm
B)1840 nm
C)1150 nm
D)1340 nm
E)203 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
63
The wavelength of coherent ruby laser light is 694 nm.What energy difference exists between the upper excited state involved and the lower unexcited ground state? (h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10−19 J,and 1 nm = 10−9 m)
A)0.56 eV
B)1.79 eV
C)1.67 eV
D)2.87 eV
E)0.35 eV
A)0.56 eV
B)1.79 eV
C)1.67 eV
D)2.87 eV
E)0.35 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
64
For which of the following transitions in a hydrogen atom free of external fields does the electron energy increase?
A)The electron moves from n = 2 to n = 1.
B)The electron moves from n = 3 to n = 5.
C)Within the n = 4,l = 2 sublevel,the electron moves from = -1 to
= -1 to  = +1.
= +1.
D)Within the n = 3 level,the electron moves from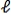 = 2 to
= 2 to 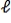 = -2.
= -2.
A)The electron moves from n = 2 to n = 1.
B)The electron moves from n = 3 to n = 5.
C)Within the n = 4,l = 2 sublevel,the electron moves from
 = -1 to
= -1 to  = +1.
= +1.D)Within the n = 3 level,the electron moves from
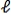 = 2 to
= 2 to 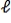 = -2.
= -2.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
65
In an x-ray machine,electrons are accelerated and then fired so that they are incident on a metal target.Which part of the process produces the characteristic x-ray spectra?
A)The incident electron loses energy.
B)The incident electron knocks an electron out of one of the metal atoms.
C)A vacancy in an energy level in a metal atom is filled.
D)The incident electron emits an x-ray.
A)The incident electron loses energy.
B)The incident electron knocks an electron out of one of the metal atoms.
C)A vacancy in an energy level in a metal atom is filled.
D)The incident electron emits an x-ray.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
66
Characteristic K x-rays are the result of:
A)inner electron transitions.
B)outer electron transitions.
C)nuclear electron states.
D)buckytubes.
A)inner electron transitions.
B)outer electron transitions.
C)nuclear electron states.
D)buckytubes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
67
The stimulated emission of photons from the excited atoms in a gas laser is prompted by which of the following?
A)high voltage
B)high flux of electrons
C)nearby presence of photons of same wavelength as those emitted
D)high temperature
A)high voltage
B)high flux of electrons
C)nearby presence of photons of same wavelength as those emitted
D)high temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
68
A ruby laser can deliver a 4.33-J pulse in approximately 50 nanoseconds.The wavelength of the light is 694 nm.At least how many atoms within the ruby rod had to be excited to allow this high-energy laser pulse? (h = 6.63 × 10−34 J⋅s,c = 3.00 × 108 m/s,1 eV = 1.6 × 10−19 J,and 1 nm = 10−9 m)
A)3.5E+18
B)3.0E+17
C)1.5E+19
D)1.5E+21
E)1.1E+18
A)3.5E+18
B)3.0E+17
C)1.5E+19
D)1.5E+21
E)1.1E+18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
69
In neon,the 20.80-eV level can undergo lasing action to the 17.43-eV level.What is the energy of the resulting photons?
A)20.80 eV
B)17.43 eV
C)38.23 eV
D)3.370 eV
E)1.685 eV
A)20.80 eV
B)17.43 eV
C)38.23 eV
D)3.370 eV
E)1.685 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
70
When a hydrogen atom absorbs a photon that raises it to the n = 5 state,how many different energies are possible for the photon(s)that may be emitted as the atom eventually returns to the ground state?
A)4
B)5
C)6
D)10
E)The correct answer is not given.
A)4
B)5
C)6
D)10
E)The correct answer is not given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
71
Characteristic x-rays are the result of
A)outer electron transitions.
B)inner electron transitions.
C)nuclear electron states.
D)buckytubes.
A)outer electron transitions.
B)inner electron transitions.
C)nuclear electron states.
D)buckytubes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the following conditions must be satisfied for laser action?
A)A ruby or similar crystalline material must be used.
B)A population inversion must occur.
C)The photons must be red.
D)A binary system must be used.
A)A ruby or similar crystalline material must be used.
B)A population inversion must occur.
C)The photons must be red.
D)A binary system must be used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
73
When a hydrogen atom absorbs a photon that raises it to the n = 7 state,what is the greatest number of photons that can be emitted by that atom as it returns to the ground state?
A)6
B)7
C)8
D)14
E)The correct answer is not given.
A)6
B)7
C)8
D)14
E)The correct answer is not given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck








