Deck 42: Molecules and Condensed Matter
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/31
العب
ملء الشاشة (f)
Deck 42: Molecules and Condensed Matter
1
A rotating diatomic molecule has rotational quantum number l.The energy DIFFERENCE between adjacent energy levels
A) increases as l increases.
B) decreases as l increases.
C) is the same for all changes in l.
D) is independent of l.
A) increases as l increases.
B) decreases as l increases.
C) is the same for all changes in l.
D) is independent of l.
increases as l increases.
2
The vibrational frequency of an HF molecule is 8.72 × 1013 Hz and the reduced mass of the molecule is 1.589 × 10-27 kg.What is the ground state vibrational energy of an HF molecule? (1 eV = 1.60 × 10-19 J,h = 1.055 × 10-34 J • s,h = 6.626 × 10-34 J • s)
A) 0.12 eV
B) 0.18 eV
C) 0.24 eV
D) 0.30 eV
E) 0.36 eV
A) 0.12 eV
B) 0.18 eV
C) 0.24 eV
D) 0.30 eV
E) 0.36 eV
0.18 eV
3
The moment of inertia of a fluorine ( 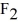 )molecule is
)molecule is 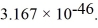 What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
A) 4.2 × 10-2 eV
B) 2.1 × 10-3 eV
C) 4.6 × 10-2 eV
D) 5.1 × 10-2 eV
E) 5.6 × 10-2 eV
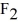 )molecule is
)molecule is 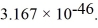 What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)A) 4.2 × 10-2 eV
B) 2.1 × 10-3 eV
C) 4.6 × 10-2 eV
D) 5.1 × 10-2 eV
E) 5.6 × 10-2 eV
4.2 × 10-2 eV
4
Estimate the rotational energy (in eV)for a diatomic hydrogen molecule in the l = 2 quantum state.(The equilibrium separation for the H2 molecule is 0.074 nm.) 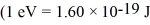 ,
, 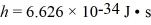 ,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)
A) 0.011 eV
B) 0.026 eV
C) 0.032 eV
D) 0.046 eV
E) 0.055 eV
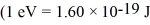 ,
, 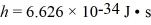 ,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mh ≈ mproton = 1.67 × 10-27 kg)A) 0.011 eV
B) 0.026 eV
C) 0.032 eV
D) 0.046 eV
E) 0.055 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
5
A vibrating diatomic molecule in its ground state has energy E.What is the energy of the same molecule in its second EXCITED state?
A) E
B) 2E
C) 3E
D) 5E
E) 9E
A) E
B) 2E
C) 3E
D) 5E
E) 9E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
6
A certain molecule has 2.00 eV of rotational energy in the l = 1 state.In the l = 4 state,what would its rotational energy be?
A) 8.00 eV
B) 16.0 eV
C) 20.0 eV
D) 30.0 eV
E) 32.0 eV
A) 8.00 eV
B) 16.0 eV
C) 20.0 eV
D) 30.0 eV
E) 32.0 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
7
A rotating diatomic molecule in its l = 1 quantum state has energy E.What is the energy of the same molecule in its l = 2 quantum state?
A) 2E
B) 3E
C) 4E
D) 6E
E) 8E
A) 2E
B) 3E
C) 4E
D) 6E
E) 8E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
8
In a p-type semiconductor,a hole is
A) a donor atom.
B) an extra electron supplied by a donor atom.
C) an extra proton supplied by a donor atom.
D) a missing atom in the crystalline structure.
E) a region where an electron is missing.
A) a donor atom.
B) an extra electron supplied by a donor atom.
C) an extra proton supplied by a donor atom.
D) a missing atom in the crystalline structure.
E) a region where an electron is missing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
9
A diatomic molecule has 18 × 10-5 eV of rotational energy in the l = 2 quantum state.What is its rotational energy in the l = 0 quantum state?
A) 90 µeV
B) 60 µeV
C) 30 µeV
D) 15 µeV
E) 0 eV
A) 90 µeV
B) 60 µeV
C) 30 µeV
D) 15 µeV
E) 0 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
10
A diatomic molecule has 2.6 × 10-5 eV of rotational energy in the l = 2 quantum state.What is its rotational energy in the l = 1 quantum state?
A) 3.4 µeV
B) 4.1 µeV
C) 5.3 µeV
D) 7.8 µeV
E) 8.7 µeV
A) 3.4 µeV
B) 4.1 µeV
C) 5.3 µeV
D) 7.8 µeV
E) 8.7 µeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
11
A diatomic molecule is vibrating in its first excited quantum state above the ground state.In that excited state,its frequency is 2.0 × 1013 Hz.What is the energy of the molecule in this state? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J
A) 0.041 eV
B) 0.083 eV
C) 0.12 eV
D) 0.15 eV
E) 0.17 eV
A) 0.041 eV
B) 0.083 eV
C) 0.12 eV
D) 0.15 eV
E) 0.17 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
12
An unfilled electron state in the valence band is called
A) a hole.
B) an empty electron.
C) a conduction electron.
D) a positron.
E) an empty positron.
A) a hole.
B) an empty electron.
C) a conduction electron.
D) a positron.
E) an empty positron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
13
Ionic bonding is due to
A) the sharing of electrons between atoms.
B) the transfer of electrons between atoms.
C) atoms bonding to hydrogen molecules.
D) atoms bonding to oxygen molecules.
A) the sharing of electrons between atoms.
B) the transfer of electrons between atoms.
C) atoms bonding to hydrogen molecules.
D) atoms bonding to oxygen molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
14
A vibrating diatomic molecule has vibrational quantum number n.The energy DIFFERENCE between adjacent energy levels
A) increases as n increases.
B) decreases as n increases.
C) is the same for all changes in n.
D) is independent of n.
A) increases as n increases.
B) decreases as n increases.
C) is the same for all changes in n.
D) is independent of n.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
15
A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state.What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 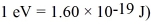
A) 1.93 × 1014 Hz
B) 2.90 × 1014 Hz
C) 4.35 × 1014 Hz
D) 1.82 × 1015 Hz
E) 2.73 × 1015 Hz
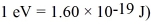
A) 1.93 × 1014 Hz
B) 2.90 × 1014 Hz
C) 4.35 × 1014 Hz
D) 1.82 × 1015 Hz
E) 2.73 × 1015 Hz

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
16
Covalent bonding is due to
A) the sharing of electrons between atoms.
B) the transfer of electrons between atoms.
C) atoms bonding to hydrogen molecules.
D) atoms bonding to oxygen molecules.
A) the sharing of electrons between atoms.
B) the transfer of electrons between atoms.
C) atoms bonding to hydrogen molecules.
D) atoms bonding to oxygen molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
17
The spacing of the atoms (treated as point masses)in the H2 molecule is 7.4 × 10-11 m.What is the energy of the l = 1 rotational level? (1 eV = 1.60 × 10-19 J, 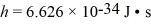 , h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)
, h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)
A) 0.090 eV
B) 0.070 eV
C) 0.045 eV
D) 0.030 eV
E) 0.015 eV
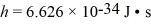 , h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)
, h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)A) 0.090 eV
B) 0.070 eV
C) 0.045 eV
D) 0.030 eV
E) 0.015 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
18
When a certain diatomic molecule undergoes a transition from the l = 5 to the l = 3 rotational level,the emitted photon has wavelength 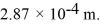 Calculate the moment of inertia of the molecule.(c = 3.00 × 108 m/s,e = 1.60 × 10-19 C,h = 6.626 × 10-34 J • s,
Calculate the moment of inertia of the molecule.(c = 3.00 × 108 m/s,e = 1.60 × 10-19 C,h = 6.626 × 10-34 J • s,
h = 1.055 × 10-34 J • s)
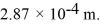 Calculate the moment of inertia of the molecule.(c = 3.00 × 108 m/s,e = 1.60 × 10-19 C,h = 6.626 × 10-34 J • s,
Calculate the moment of inertia of the molecule.(c = 3.00 × 108 m/s,e = 1.60 × 10-19 C,h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
19
A p-type semiconductor has a net positive charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
20
A diatomic has a moment of inertia of 7.73 × 10-45 kg∙ m2.What is its rotational energy in the quantum state characterized by l = 2? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 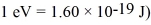
A) 22.7 µeV
B) 27.0 µeV
C) 587 µeV
D) 72.2 µeV
E) 87.1 µeV
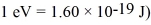
A) 22.7 µeV
B) 27.0 µeV
C) 587 µeV
D) 72.2 µeV
E) 87.1 µeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
21
The energy gap between the valence and conduction bands in a certain semiconductor is 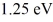 .What is the threshold wavelength for optical absorption in this substance?
.What is the threshold wavelength for optical absorption in this substance? 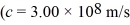 ,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)
,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)
A) 599 nm
B) 639 nm
C) 959 nm
D) 873 nm
E) 994 nm
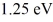 .What is the threshold wavelength for optical absorption in this substance?
.What is the threshold wavelength for optical absorption in this substance? 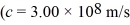 ,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)
,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)A) 599 nm
B) 639 nm
C) 959 nm
D) 873 nm
E) 994 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
22
Silver has a Fermi level (energy)of 5.5 eV.At 0 K,at what speed would the electrons be moving if they had kinetic energy equal to the Fermi energy? (This speed is known as the Fermi speed.)(mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)
A) 1400 km/s
B) 1.0 km/s
C) 2.2 km/s
D) 1100 km/s
E) 3.5 km/s
A) 1400 km/s
B) 1.0 km/s
C) 2.2 km/s
D) 1100 km/s
E) 3.5 km/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
23
If one metal has double the number of conduction electrons per unit volume of a second metal,then its Fermi level (energy)is how many times that of the second metal?
A) 1.41
B) 2.83
C) 2.00
D) 3.22
E) 1.59
A) 1.41
B) 2.83
C) 2.00
D) 3.22
E) 1.59

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
24
The Fermi level (energy)of a metal is 5.5 eV.What is the number of conduction electrons per unit volume for this metal? (mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J, 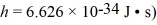
A) 5.8 × 1028 / m3
B) 6.2 × 1028 / m3
C) 4.1 × 1028 / m3
D) 8.4 × 1028 / m3
E) 3.3 × 1028 / m3
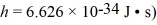
A) 5.8 × 1028 / m3
B) 6.2 × 1028 / m3
C) 4.1 × 1028 / m3
D) 8.4 × 1028 / m3
E) 3.3 × 1028 / m3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
25
A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state.If the molecule made a transition from the n = 2 state to the n = 1 state,what would be the energy of the photon it would emit? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
A) 3.60 eV
B) 2.40 eV
C) 1.20 eV
D) 0.60 eV
E) 0.30 eV
A) 3.60 eV
B) 2.40 eV
C) 1.20 eV
D) 0.60 eV
E) 0.30 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
26
The Fermi energy of rubidium at a temperature of 5 K is 1.85 eV.An electron state in rubidium is 0.007 eV above the Fermi level.What is the probability that this state is occupied at a temperature of 9K? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s,Boltzmann constant = 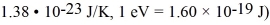
A) 1 × 10-4
B) 2 × 10-5
C) 5 × 10-6
D) 1 × 10-6
E) 2 × 10-7
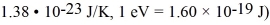
A) 1 × 10-4
B) 2 × 10-5
C) 5 × 10-6
D) 1 × 10-6
E) 2 × 10-7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the occupancy probability at an energy of 12.00 eV for a material with a Fermi energy (level)of 11.63 eV at a temperature of 500 K? (mel = 9.11 × 10-31 kg, 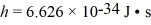 ,Boltzmann constant = 1.38 × 10-23,1 eV = 1.60 × 10-19 J)
,Boltzmann constant = 1.38 × 10-23,1 eV = 1.60 × 10-19 J)
A) 0.00030%
B) 0.019%
C) 2.0%
D) 6.0%
E) 12%
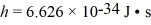 ,Boltzmann constant = 1.38 × 10-23,1 eV = 1.60 × 10-19 J)
,Boltzmann constant = 1.38 × 10-23,1 eV = 1.60 × 10-19 J)A) 0.00030%
B) 0.019%
C) 2.0%
D) 6.0%
E) 12%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
28
A metal has a Fermi level (energy)of 5.50 eV.At 1200 K,what energy will have a 90% occupancy probability? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s,Boltzmann constant = 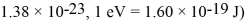
A) 5.79 eV
B) 5.73 eV
C) 5.56 eV
D) 5.44 eV
E) 5.27 eV
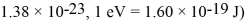
A) 5.79 eV
B) 5.73 eV
C) 5.56 eV
D) 5.44 eV
E) 5.27 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
29
If a metal had a Fermi level (energy)of 5.0 eV,what would be the average energy of the electrons at 0 K?
A) 8.0 eV
B) 6.0 eV
C) 3.0 eV
D) 0.00 eV
E) 5.0 eV
A) 8.0 eV
B) 6.0 eV
C) 3.0 eV
D) 0.00 eV
E) 5.0 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
30
For a solid in which the occupation of the energy states is given by the Fermi-Dirac distribution,the probability that a certain state is occupied at a temperature T0 is 0.70.If the temperature is doubled to 2T0,what is the probability that the same state is occupied? Assume that the Fermi energy does not change with temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck
31
Approximately how many states in the range from 5.0 eV to 5.2 eV are there in a copper bar of volume 5.3 cm3? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 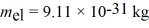 ,
, 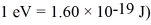
A) 5.1 × 1022
B) 3.2 × 1022
C) 1.6 × 1022
D) 8.2 × 1021
E) 3.2 × 1021
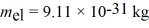 ,
, 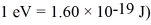
A) 5.1 × 1022
B) 3.2 × 1022
C) 1.6 × 1022
D) 8.2 × 1021
E) 3.2 × 1021

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 31 في هذه المجموعة.
فتح الحزمة
k this deck








