Deck 13: Solutions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/158
العب
ملء الشاشة (f)
Deck 13: Solutions
1
To increase solubility of a gas into a liquid the most,then
A) increase the temperature and lower the pressure.
B) increase the temperature and raise the pressure.
C) decrease the temperature and raise the pressure.
D) decrease the temperature and lower the pressure.
E) neither pressure or temperature affects solubility.
A) increase the temperature and lower the pressure.
B) increase the temperature and raise the pressure.
C) decrease the temperature and raise the pressure.
D) decrease the temperature and lower the pressure.
E) neither pressure or temperature affects solubility.
decrease the temperature and raise the pressure.
2
________ is the gas dissolved in carbonated drinks.
A) Carbon dioxide
B) Nitrogen
C) Helium
D) Hydrogen
E) Oxygen
A) Carbon dioxide
B) Nitrogen
C) Helium
D) Hydrogen
E) Oxygen
Carbon dioxide
3
A solution containing less than the equilibrium amount is called
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.
an unsaturated solution.
4
To make rock candy,sugar is dissolved in hot water until no more can dissolve,then the solution is allowed to cool.A stick is placed in the sugar solution and crystals form on the stick.Describes what happens in terms of unsaturation,saturation,and supersaturation.
A) The hot solution is saturated and the cooled solution is unsaturated.
B) The hot solution is supersaturated and the cooled solution is saturated.
C) The hot solution is unsaturated and the cooled solution is saturated.
D) The hot solution is saturated and the cooled solution is supersaturated.
E) The hot solution is supersaturated and the cooled solution is unsaturated.
A) The hot solution is saturated and the cooled solution is unsaturated.
B) The hot solution is supersaturated and the cooled solution is saturated.
C) The hot solution is unsaturated and the cooled solution is saturated.
D) The hot solution is saturated and the cooled solution is supersaturated.
E) The hot solution is supersaturated and the cooled solution is unsaturated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
5
Identify the major force between molecules of pentane.
A) dipole-dipole
B) dispersion
C) hydrogen bonding
D) ion-ion
E) ion-dipole
A) dipole-dipole
B) dispersion
C) hydrogen bonding
D) ion-ion
E) ion-dipole

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
6
A solution in which the dissolved solute is in dynamic equilibrium with the solid solute is called
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements is generally TRUE?
A) The solubility of a solid is not dependent on either temperature or pressure.
B) The solubility of a solid is highly dependent on pressure.
C) The solubility of a solid is highly dependent on both pressure and temperature.
D) The solubility of a solid is highly dependent on temperature.
E) None of the above.
A) The solubility of a solid is not dependent on either temperature or pressure.
B) The solubility of a solid is highly dependent on pressure.
C) The solubility of a solid is highly dependent on both pressure and temperature.
D) The solubility of a solid is highly dependent on temperature.
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
8
Choose the situation below that would result in a ΔHsolution near 0.
A) When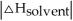 >>
>>
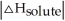
B) When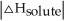 >
>
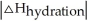
C) When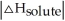 <
<
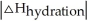
D) When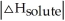 is close to
is close to
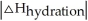
E) There isn't enough information to determine.
A) When
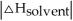 >>
>>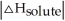
B) When
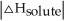 >
>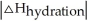
C) When
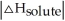 <
<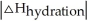
D) When
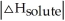 is close to
is close to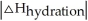
E) There isn't enough information to determine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
9
Give the major force between acetone and chloroform.
A) dipole-dipole
B) dispersion
C) hydrogen bonding
D) ion-ion
E) ion-dipole
A) dipole-dipole
B) dispersion
C) hydrogen bonding
D) ion-ion
E) ion-dipole

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
10
Define solubility.
A) a solid that does not dissolve in a gas
B) the amount of a substance that will dissolve in a given amount of solvent
C) the amount of a substance that will dissolve in a given amount of solute
D) a solid mixed with another solid
E) a liquid that does not dissolve in another liquid
A) a solid that does not dissolve in a gas
B) the amount of a substance that will dissolve in a given amount of solvent
C) the amount of a substance that will dissolve in a given amount of solute
D) a solid mixed with another solid
E) a liquid that does not dissolve in another liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following compounds is most soluble in hexane (CH3CH2CH2CH2CH2CH2CH3)?
A) methanol
B) ethanol
C) 1-propanol
D) 1-butanol
E) 1-pentanol
A) methanol
B) ethanol
C) 1-propanol
D) 1-butanol
E) 1-pentanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
12
Describe what happens when seawater is consumed to quench thirst.
A) Seawater can quench thirst once it is boiled.
B) Seawater quenches thirst when directly ingested.
C) Seawater draws water out of the body resulting in further dehydration and diarrhea.
D) Seawater helps diarrhea.
E) Seawater must be ingested at twice the volume to quench thirst.
A) Seawater can quench thirst once it is boiled.
B) Seawater quenches thirst when directly ingested.
C) Seawater draws water out of the body resulting in further dehydration and diarrhea.
D) Seawater helps diarrhea.
E) Seawater must be ingested at twice the volume to quench thirst.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
13
If two substances are soluble in all proportions then they are ________ in each other.
A) miscible
B) nonpolar
C) immiscible
D) neutral
E) polar
A) miscible
B) nonpolar
C) immiscible
D) neutral
E) polar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
14
Determine ΔHsolute for KBr if the ΔHsolution (KBr)= +19.9 kJ/mol and the ΔHhydration(KBr)= -670.kJ/mol.
A) +650 kJ/mol
B) -650 kJ/mol
C) +690 kJ/mol
D) -710 kJ/mol
E) -690 kJ/mol
A) +650 kJ/mol
B) -650 kJ/mol
C) +690 kJ/mol
D) -710 kJ/mol
E) -690 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
15
Choose the situation below that would result in an exothermic ΔHsolution.
A) When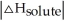 >
>
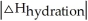
B) When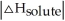 is close to
is close to
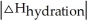
C) When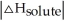 <
<
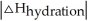
D) When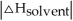 >>
>>
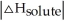
E) There isn't enough information to determine.
A) When
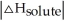 >
>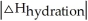
B) When
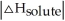 is close to
is close to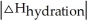
C) When
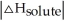 <
<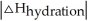
D) When
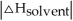 >>
>>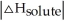
E) There isn't enough information to determine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
16
A solution containing more than the equilibrium amount is called
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.
A) an unsaturated solution.
B) a dilute solution.
C) a supersaturated solution.
D) a concentrated solution.
E) a saturated solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following compounds will be most soluble in ethanol (CH3CH2OH)?
A) trimethylamine (N(CH3)3)
B) acetone (CH3COCH3)
C) ethylene glycol (HOCH2CH2OH)
D) hexane (CH3CH2CH2CH2CH2CH3)
E) None of these compounds should be soluble in ethanol.
A) trimethylamine (N(CH3)3)
B) acetone (CH3COCH3)
C) ethylene glycol (HOCH2CH2OH)
D) hexane (CH3CH2CH2CH2CH2CH3)
E) None of these compounds should be soluble in ethanol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
18
A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some solid remains at the bottom of the solution.Which statement below is TRUE?
A) The solution is considered unsaturated.
B) The solution is considered supersaturated.
C) The solution is considered saturated.
D) The solution would be considered unsaturated if it were cooled a bit to increase the solubility of the solid.
E) None of the above is true.
A) The solution is considered unsaturated.
B) The solution is considered supersaturated.
C) The solution is considered saturated.
D) The solution would be considered unsaturated if it were cooled a bit to increase the solubility of the solid.
E) None of the above is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
19
Choose the statement below that is TRUE.
A) A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions.
B) A solution will form between two substances if the solute-solvent interactions are small enough to be overcome by the solute-solute and solvent-solvent interactions.
C) A solution will form between two substances if the solute-solute interactions are strong enough to overcome the solvent-solvent interactions.
D) A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions.
E) None of the above is true.
A) A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions.
B) A solution will form between two substances if the solute-solvent interactions are small enough to be overcome by the solute-solute and solvent-solvent interactions.
C) A solution will form between two substances if the solute-solute interactions are strong enough to overcome the solvent-solvent interactions.
D) A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions.
E) None of the above is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
20
Choose the situation below that would result in an endothermic ΔHsolution.
A) When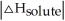 >
>
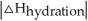
B) When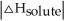 <
<
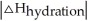
C) When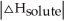 is close to
is close to
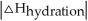
D) When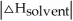 >>
>>
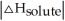
E) There isn't enough information to determine.
A) When
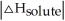 >
>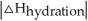
B) When
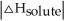 <
<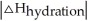
C) When
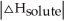 is close to
is close to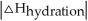
D) When
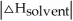 >>
>>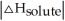
E) There isn't enough information to determine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
21
Commercial grade HCl solutions are typically 39.0% (by mass)HCl in water.Determine the molarity of the HCl,if the solution has a density of 1.20 g/mL.
A) 7.79 M
B) 10.7 M
C) 12.8 M
D) 9.35 M
E) 13.9 M
A) 7.79 M
B) 10.7 M
C) 12.8 M
D) 9.35 M
E) 13.9 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
22
The Dirty Dozen refer to twelve chemicals that are harmful for the environment.Identify the compound that was not used as an insecticide.
A) Chlordane
B) DDT
C) Aldrin
D) polychlorinated biphenyl
E) Heptachlor
A) Chlordane
B) DDT
C) Aldrin
D) polychlorinated biphenyl
E) Heptachlor

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
23
A solution is prepared by dissolving 76.3 g NaI in 545 g of water.Determine the mole fraction of NaI if the final volume of the solution is 576 mL.
A) 6.04 × 10-3
B) 1.65 × 10-2
C) 1.40 × 10-3
D) 1.32 × 10-2
E) 8.84 × 10-2
A) 6.04 × 10-3
B) 1.65 × 10-2
C) 1.40 × 10-3
D) 1.32 × 10-2
E) 8.84 × 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following concentration units is temperature dependent?
A) mole fraction
B) molality
C) mass percent
D) molarity
E) none of the above
A) mole fraction
B) molality
C) mass percent
D) molarity
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
25
______ is burped up from the bottom of a water-filled volcanic crater.
A) Carbon monoxide
B) Oxygen
C) Nitrogen
D) Hydrogen
E) Carbon dioxide
A) Carbon monoxide
B) Oxygen
C) Nitrogen
D) Hydrogen
E) Carbon dioxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
26
Give the term for the amount of solute in moles per liter of solution.
A) molality
B) molarity
C) mole fraction
D) mole percent
E) mass percent
A) molality
B) molarity
C) mole fraction
D) mole percent
E) mass percent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
27
A solution is prepared by dissolving 38.6 g sucrose (C12H22O11)in 495 g of water.Determine the mole fraction of sucrose if the final volume of the solution is 508 mL.
A) 4.09 × 10-3
B) 7.80 × 10-2
C) 1.28 × 10-3
D) 7.23 × 10-2
E) 2.45 × 10-3
A) 4.09 × 10-3
B) 7.80 × 10-2
C) 1.28 × 10-3
D) 7.23 × 10-2
E) 2.45 × 10-3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements is TRUE?
A) In general, the solubility of a solid in water decreases with increasing temperature.
B) In general, the solubility of a gas in water decreases with increasing temperature.
C) The solubility of a gas in water usually increases with decreasing pressure.
D) The solubility of an ionic solid in water decreases with increasing temperature.
E) None of the above statements is true.
A) In general, the solubility of a solid in water decreases with increasing temperature.
B) In general, the solubility of a gas in water decreases with increasing temperature.
C) The solubility of a gas in water usually increases with decreasing pressure.
D) The solubility of an ionic solid in water decreases with increasing temperature.
E) None of the above statements is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
29
Calculate the mass of oxygen (in mg)dissolved in a 5.00 L bucket of water exposed to a pressure of 1.13 atm of air.Assume the mole fraction of oxygen in air to be 0.21 and the Henry's law constant for oxygen in water at this temperature to be 1.3 × 10-3 M/atm.
A) 49.4 mg
B) 23.5 mg
C) 9.87 mg
D) 27.3 mg
E) 13.7 mg
A) 49.4 mg
B) 23.5 mg
C) 9.87 mg
D) 27.3 mg
E) 13.7 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
30
A solid can be purified through what technique?
A) recrystallization
B) dilution
C) dissolution
D) boiling
E) melting
A) recrystallization
B) dilution
C) dissolution
D) boiling
E) melting

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
31
What mass (in g)of NH3 must be dissolved in 475 g of methanol to make a 0.250 m solution?
A) 2.02 g
B) 4.94 g
C) 1.19 g
D) 8.42 g
E) 1.90 g
A) 2.02 g
B) 4.94 g
C) 1.19 g
D) 8.42 g
E) 1.90 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
32
Identify the colligative property.
A) vapor pressure lowering
B) freezing point depression
C) boiling point elevation
D) osmotic pressure
E) all of the above
A) vapor pressure lowering
B) freezing point depression
C) boiling point elevation
D) osmotic pressure
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
33
0.300 moles of sodium nitrite are needed for a reaction.The solution is 0.450 M.How many mL are needed?
A) 0.667 mL
B) 1500 mL
C) 1.5 mL
D) 667 mL
E) 135 mL
A) 0.667 mL
B) 1500 mL
C) 1.5 mL
D) 667 mL
E) 135 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
34
Give the term for the amount of solute in moles per kilogram of solvent.
A) molality
B) molarity
C) mole fraction
D) mole percent
E) mass percent
A) molality
B) molarity
C) mole fraction
D) mole percent
E) mass percent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
35
Calculate the number of moles of sulfuric acid that is contained in 0.250 mL of 0.500 M sulfuric acid solution.
A) 0.125 moles
B) 2.00 moles
C) 0.500 moles
D) 8.00 moles
E) 0.600 moles
A) 0.125 moles
B) 2.00 moles
C) 0.500 moles
D) 8.00 moles
E) 0.600 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
36
Calculate the molality of a solution that is prepared by mixing 25.5 mL of CH3OH (d = 0.792 g/mL)and 387 mL of CH3CH2CH2OH (d = 0.811 g/mL).
A) 0.630 m
B) 0.812 m
C) 1.57 m
D) 2.01 m
E) 4.98 m
A) 0.630 m
B) 0.812 m
C) 1.57 m
D) 2.01 m
E) 4.98 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
37
Choose the solvent below that would show the greatest freezing point lowering when used to make a 0.20 m nonelectrolyte solution.
A) carbon tetrachloride, Kf = 29.9°C/m
B) chloroform, Kf = 4.70°C/m
C) benzene, Kf = 5.12°C/m
D) ethanol, Kf = 1.99°C/m
E) diethyl ether, Kf = 1.79°C/m
A) carbon tetrachloride, Kf = 29.9°C/m
B) chloroform, Kf = 4.70°C/m
C) benzene, Kf = 5.12°C/m
D) ethanol, Kf = 1.99°C/m
E) diethyl ether, Kf = 1.79°C/m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
38
Commercial grade HCl solutions are typically 39.0% (by mass)HCl in water.Determine the molality of the HCl,if the solution has a density of 1.20 g/mL.
A) 39.0 m
B) 17.5 m
C) 6.39 m
D) 10.7 m
E) 9.44 m
A) 39.0 m
B) 17.5 m
C) 6.39 m
D) 10.7 m
E) 9.44 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
39
Determine the Henry's law constant for ammonia in water at 25°C if an ammonia pressure of 0.022 atm produces a solution with a concentration of 1.3 M.
A) 59 M/atm
B) 0.017 M/atm
C) 0.029 M/atm
D) 35 M/atm
E) 0.038 M/atm
A) 59 M/atm
B) 0.017 M/atm
C) 0.029 M/atm
D) 35 M/atm
E) 0.038 M/atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
40
Determine the vapor pressure of a solution at 25°C that contains 76.6 g of glucose (C6H12O6)in 250.0 mL of water.The vapor pressure of pure water at 25°C is 23.8 torr.
A) 70.8 torr
B) 72.9 torr
C) 23.1 torr
D) 22.9 torr
E) 7.29 torr
A) 70.8 torr
B) 72.9 torr
C) 23.1 torr
D) 22.9 torr
E) 7.29 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
41
Identify the classification of milk.
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
42
Identify the classification of opal.
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
43
Define colloid.
A) a mixture in which a substance floats on the dispersing medium
B) a mixture in which a substance is dissolved in a solute
C) a mixture in which a substance is dissolved in a solvent
D) a mixture in which a substance settles to the bottom of a dispersing medium
E) a mixture in which a dispersed substance is finely divided in a dispersing medium
A) a mixture in which a substance floats on the dispersing medium
B) a mixture in which a substance is dissolved in a solute
C) a mixture in which a substance is dissolved in a solvent
D) a mixture in which a substance settles to the bottom of a dispersing medium
E) a mixture in which a dispersed substance is finely divided in a dispersing medium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
44
Solutions having osmotic pressures more than those of body fluids are called
A) isosmotic.
B) hyperosmotic.
C) hyposmotic.
D) neosmotic.
E) magnosmotic.
A) isosmotic.
B) hyperosmotic.
C) hyposmotic.
D) neosmotic.
E) magnosmotic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
45
The boiling point elevation of an aqueous sucrose solution is found to be 0.39°C.What mass of sucrose (molar mass= 342.30 g/mol)would be needed to dissolve in 500.0 g of water? Kb (water)= 0.512°C/m.
A) 261 g sucrose
B) 528 g sucrose
C) 762 g sucrose
D) 223 g sucrose
E) 130. g sucrose
A) 261 g sucrose
B) 528 g sucrose
C) 762 g sucrose
D) 223 g sucrose
E) 130. g sucrose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
46
Calculate the freezing point of a solution of 500.0 g of ethylene glycol (C2H6O2)dissolved in 500.0 g of water.Kf = 1.86°C/m and Kb = 0.512°C/m.
A) 30.0°C
B) -30.0°C
C) 8.32°C
D) -8.32°C
E) 70.2°C
A) 30.0°C
B) -30.0°C
C) 8.32°C
D) -8.32°C
E) 70.2°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
47
Determine the vapor pressure of a solution at 55°C that contains 34.2 g NaCl in 375 mL of water.The vapor pressure of pure water at 55°C is 118.1 torr.The van't Hoff factor for NaCl is 1.9.
A) 115 torr
B) 87.1 torr
C) 108 torr
D) 112 torr
E) 92.8 torr
A) 115 torr
B) 87.1 torr
C) 108 torr
D) 112 torr
E) 92.8 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
48
Identify the classification of whipped cream.
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion
A) aerosol
B) solid aerosol
C) foam
D) emulsion
E) solid emulsion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
49
A compound is found to have a molar mass of 598 g/mol.If 35.8 mg of the compound is dissolved in enough water to make 175 mL of solution at 25°C,what is the osmotic pressure of the resulting solution?
A) 3.42 torr
B) 6.36 torr
C) 5.01 torr
D) 5.99 torr
E) 8.36 torr
A) 3.42 torr
B) 6.36 torr
C) 5.01 torr
D) 5.99 torr
E) 8.36 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
50
The boiling point of an aqueous 1.83 m (NH4)2SO4 (molar mass = 132.15 g/mol)solution is 102.5°C.Determine the value of the van't Hoff factor for this solute if the Kb for water is 0.512°C/m.
A) 3.0
B) 3.6
C) 1.8
D) 2.7
E) 2.3
A) 3.0
B) 3.6
C) 1.8
D) 2.7
E) 2.3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
51
Give the reason that antifreeze is added to a car radiator.
A) The freezing point is lowered and the boiling point is elevated.
B) The freezing point is elevated and the boiling point is lowered.
C) The freezing point and the boiling point are elevated.
D) The freezing point and the boiling point are lowered.
E) None of the above
A) The freezing point is lowered and the boiling point is elevated.
B) The freezing point is elevated and the boiling point is lowered.
C) The freezing point and the boiling point are elevated.
D) The freezing point and the boiling point are lowered.
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
52
Determine the freezing point depression of a solution that contains 30.7 g glycerin (C3H8O3,molar mass = 92.09 g/mol)in 376 mL of water.Some possibly useful constants for water are Kf = 1.86°C/m and Kb = 0.512°C/m.
A) 0.887°C
B) 1.65°C
C) 3.33°C
D) 4.98°C
E) 0.654°C
A) 0.887°C
B) 1.65°C
C) 3.33°C
D) 4.98°C
E) 0.654°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
53
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (C2H6O2)dissolved in 500.0 g of water.Kf = 1.86°C/m and Kb = 0.512°C/m.Use 100°C as the boiling point of water.
A) 108°C
B) 92°C
C) 130°C
D) 70°C
E) 8.3°C
A) 108°C
B) 92°C
C) 130°C
D) 70°C
E) 8.3°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
54
Fluids used for an intravenous transfusion must be ________ with bodily fluids.
A) isosmotic
B) hyperosmotic
C) hyposmotic
D) neosmotic
E) magnosmotic
A) isosmotic
B) hyperosmotic
C) hyposmotic
D) neosmotic
E) magnosmotic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
55
Determine the freezing point of a solution that contains 78.8 g of naphthalene (C10H8, molar mass = 128.16 g/mol)dissolved in 722 mL of benzene (d = 0.877 g/mL).Pure benzene has a melting point of 5.50°C and a freezing point depression constant of 4.90°C/m.
A) 4.76°C
B) 4.17°C
C) 0.74°C
D) 1.33°C
E) 1.68°C
A) 4.76°C
B) 4.17°C
C) 0.74°C
D) 1.33°C
E) 1.68°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
56
Solutions having osmotic pressures less than those of body fluids are called
A) isosmotic.
B) hyperosmotic.
C) hyposmotic.
D) neosmotic.
E) magnosmotic.
A) isosmotic.
B) hyperosmotic.
C) hyposmotic.
D) neosmotic.
E) magnosmotic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
57
Determine the boiling point of a solution that contains 78.8 g of naphthalene (C10H8, molar mass = 128.16 g/mol)dissolved in 722 mL of benzene (d = 0.877 g/mL).Pure benzene has a boiling point of 80.1°C and a boiling point elevation constant of 2.53°C/m.
A) 2.2°C
B) 2.5°C
C) 82.3°C
D) 80.4°C
E) 82.6°C
A) 2.2°C
B) 2.5°C
C) 82.3°C
D) 80.4°C
E) 82.6°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
58
Soap has an ionic and a polar end.It works well to remove oil by
A) surrounding the oil with the nonpolar end, and the water interacts with the polar end.
B) surrounding the oil with the polar end, and the water interacts with the nonpolar end.
C) surrounding the oil and water with the polar end.
D) surrounding the oil and water with the nonpolar end.
A) surrounding the oil with the nonpolar end, and the water interacts with the polar end.
B) surrounding the oil with the polar end, and the water interacts with the nonpolar end.
C) surrounding the oil and water with the polar end.
D) surrounding the oil and water with the nonpolar end.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
59
A 150.0 mL sample of an aqueous solution at 25°C contains 15.2 mg of an unknown nonelectrolyte compound.If the solution has an osmotic pressure of 8.44 torr,what is the molar mass of the unknown compound?
A) 223 g/mol
B) 294 g/mol
C) 341 g/mol
D) 448 g/mol
E) 195 g/mol
A) 223 g/mol
B) 294 g/mol
C) 341 g/mol
D) 448 g/mol
E) 195 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
60
Light beams that are visible in a dusty room is an example of
A) an emulsion.
B) an aerosol.
C) micelle repulsion.
D) Brownian motion.
E) Tyndall effect.
A) an emulsion.
B) an aerosol.
C) micelle repulsion.
D) Brownian motion.
E) Tyndall effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
61
Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.6 atm.The Henry's law constant for carbon dioxide in water at this temperature is 3.4 × 10-2 M/atm.
A) 0.16 M
B) 0.53 M
C) 0.61 M
D) 0.19 M
E) 0.78 M
A) 0.16 M
B) 0.53 M
C) 0.61 M
D) 0.19 M
E) 0.78 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
62
Dissolving of a salt can be defined as
A) solvation of ions, with no change in the amount of solid.
B) solvation of ions, with increase in the amount of solid.
C) solvation of ions, with decrease in the amount of solid.
D) rate of bubbling > rate of dissolving.
E) rate of condensing > rate of bubbling.
A) solvation of ions, with no change in the amount of solid.
B) solvation of ions, with increase in the amount of solid.
C) solvation of ions, with decrease in the amount of solid.
D) rate of bubbling > rate of dissolving.
E) rate of condensing > rate of bubbling.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
63
Determine the partial pressure of oxygen necessary to form an aqueous solution that is 7.1 × 10-4 M O2 at 25°C.The Henry's law constant for oxygen in water at 25°C is
1)3 × 10-3 M/atm.
A) 1.1 atm
B) 0.92 atm
C) 0.88 atm
D) 1.8 atm
E) 0.55 atm
1)3 × 10-3 M/atm.
A) 1.1 atm
B) 0.92 atm
C) 0.88 atm
D) 1.8 atm
E) 0.55 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
64
Identify the compound whose solubility decreases with increasing temperature. 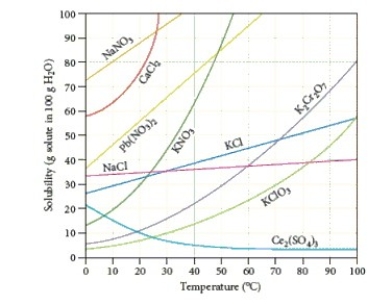
A) Ce2(SO4)3
B) K2Cr2O7
C) NaNO3
D) KClO3
E) CaCl2

A) Ce2(SO4)3
B) K2Cr2O7
C) NaNO3
D) KClO3
E) CaCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
65
Give the major force in NaCl solution.
A) dipole-dipole
B) nonpolar forces
C) hydrogen bonding
D) ion-ion
E) ion-dipole
A) dipole-dipole
B) nonpolar forces
C) hydrogen bonding
D) ion-ion
E) ion-dipole

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
66
Identify the compound whose solubility is least affected by temperature. 
A) KCl
B) K2Cr2O7
C) NaNO3
D) Pb(NO3)3
E) NaCl

A) KCl
B) K2Cr2O7
C) NaNO3
D) Pb(NO3)3
E) NaCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
67
What volume of a 0.716 M KBr solution is needed to provide 30.5 g of KBr?
A) 21.8 mL
B) 42.7 mL
C) 184 mL
D) 358 mL
A) 21.8 mL
B) 42.7 mL
C) 184 mL
D) 358 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
68
Calculate the molarity of a solution that contains 0.300 moles of CsBr in 0.600 L of water.
A) 0.500 M
B) 2.00 M
C) 0.125 M
D) 8.00 M
E) 0.750 M
A) 0.500 M
B) 2.00 M
C) 0.125 M
D) 8.00 M
E) 0.750 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
69
Identify the compounds that are soluble in both water and hexane.
A) methanol and 1-pentanol
B) ethanol and 1-butanol
C) 1-propanol and 1-butanol
D) 1-butanol and 1-pentanol
E) ethanol and 1-propanol
A) methanol and 1-pentanol
B) ethanol and 1-butanol
C) 1-propanol and 1-butanol
D) 1-butanol and 1-pentanol
E) ethanol and 1-propanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
70
Identify the most polar solvent.
A) acetone
B) octane
C) sodium chloride
D) toluene
E) carbon tetrachloride
A) acetone
B) octane
C) sodium chloride
D) toluene
E) carbon tetrachloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following compounds will be most soluble in nonane (C9H20)?
A) 1-pentanol
B) benzene
C) acetic acid
D) ethyl methyl ketone
E) ethanol
A) 1-pentanol
B) benzene
C) acetic acid
D) ethyl methyl ketone
E) ethanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
72
Dynamic equilibrium can be defined as
A) rate of dissolution = rate of recrystallization.
B) rate of dissolution < rate of recrystallization.
C) rate of dissolution > rate of recrystallization.
D) rate of bubbling > rate of dissolving.
E) rate of condensing > rate of bubbling.
A) rate of dissolution = rate of recrystallization.
B) rate of dissolution < rate of recrystallization.
C) rate of dissolution > rate of recrystallization.
D) rate of bubbling > rate of dissolving.
E) rate of condensing > rate of bubbling.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
73
Determine the solubility of N2 in water exposed to air at 25°C if the atmospheric pressure is 2.0 atm.Assume that the mole fraction of nitrogen is 0.78 in air and the Henry's law constant for nitrogen in water at this temperature is 6.1 × 10-4 M/atm.
A) 1.6 × 10-4 M
B) 6.4 × 10-4 M
C) 9.5 × 10-4 M
D) 1.1 × 10-4 M
E) 1.2 × 10-4 M
A) 1.6 × 10-4 M
B) 6.4 × 10-4 M
C) 9.5 × 10-4 M
D) 1.1 × 10-4 M
E) 1.2 × 10-4 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following should have the largest Henry's law constant (kH)in water?
A) Xe
B) CO
C) H2
D) CH3CH3
E) CO2
A) Xe
B) CO
C) H2
D) CH3CH3
E) CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which cation in each set is expected to have the larger (more negative)hydration energy? I Be2+ or Ca2+
II Rb+ or Zn2+
A) Be2+ in set I and Rb+ in set II
B) Be2+ in set I and Zn2+ in set II
C) Ca2+ in set I and Rb+ in set II
D) Ca2+ in set I and Zn2+ in set II
II Rb+ or Zn2+
A) Be2+ in set I and Rb+ in set II
B) Be2+ in set I and Zn2+ in set II
C) Ca2+ in set I and Rb+ in set II
D) Ca2+ in set I and Zn2+ in set II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
76
The Henry's law constant for helium gas in water at 30°C is 3.70 × 10-4 M/atm.When the partial pressure of helium above a sample of water is 0.650 atm,the concentration of helium in the water is ________ M.
A) 5.69 × 10-4
B) 1.76 × 103
C) 1.30
D) 2.41 × 10-4
E) 3.70 × 10-4
A) 5.69 × 10-4
B) 1.76 × 103
C) 1.30
D) 2.41 × 10-4
E) 3.70 × 10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
77
Identify the gas that is dissolved in carbonated sodas.
A) carbon dioxide
B) ammonia
C) helium
D) carbon monoxide
E) xenon
A) carbon dioxide
B) ammonia
C) helium
D) carbon monoxide
E) xenon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
78
Give the major force between ethanol and rubbing alcohol.
A) dipole-dipole
B) ionic forces
C) hydrogen bonding
D) ion-ion
E) oxygen bonding
A) dipole-dipole
B) ionic forces
C) hydrogen bonding
D) ion-ion
E) oxygen bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which of the following ions should have the most exothermic ΔHhydration?
A) Rb⁺
B) Ba2⁺
C) Al3⁺
D) Sr2⁺
E) Ni2⁺
A) Rb⁺
B) Ba2⁺
C) Al3⁺
D) Sr2⁺
E) Ni2⁺

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck
80
The Henry's Law constant of methyl bromide,CH3Br,is k = 0.159 mol/(L ∙ atm)at 25°C.What is the solubility of methyl bromide in water at 25°C and at a partial pressure of 270.mm Hg?
A) 0.0565 mol/L
B) 0.355 mol/L
C) 0.448 mol/L
D) 42.9 mol/L
A) 0.0565 mol/L
B) 0.355 mol/L
C) 0.448 mol/L
D) 42.9 mol/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 158 في هذه المجموعة.
فتح الحزمة
k this deck








