Deck 14: Chemical Kinetics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/155
العب
ملء الشاشة (f)
Deck 14: Chemical Kinetics
1
Determine the rate law and the value of k for the following reaction using the data provided. CO(g)+ Cl2(g)→ COCl2(g)[CO]i (M)[Cl2]i (M)Initial Rate (M-1s-1)
0)25 0.40 0.696
0)25 0.80 1.97
0)50 0.80 3.94
A) Rate = 11 M-3/2s-1 [CO][Cl2]3/2
B) Rate = 36 M-1.8s-1 [CO][Cl2]2.8
C) Rate = 17 M-2s-1 [CO][Cl2]2
D) Rate = 4.4 M-1/2s-1 [CO][Cl2]1/2
E) Rate = 18 M-3/2s-1 [CO]2[Cl2]1/2
0)25 0.40 0.696
0)25 0.80 1.97
0)50 0.80 3.94
A) Rate = 11 M-3/2s-1 [CO][Cl2]3/2
B) Rate = 36 M-1.8s-1 [CO][Cl2]2.8
C) Rate = 17 M-2s-1 [CO][Cl2]2
D) Rate = 4.4 M-1/2s-1 [CO][Cl2]1/2
E) Rate = 18 M-3/2s-1 [CO]2[Cl2]1/2
Rate = 11 M-3/2s-1 [CO][Cl2]3/2
2
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A]0[B]2,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
0.0200 M/s
3
Give the characteristic of a second-order reaction having only one reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.
The rate of the reaction is proportional to the square of the concentration of the reactant.
4
Give the characteristic of a first-order reaction having only one reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
5
Give the characteristic of a zero-order reaction having only one reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.
A) The rate of the reaction is not proportional to the concentration of the reactant.
B) The rate of the reaction is proportional to the square of the concentration of the reactant.
C) The rate of the reaction is proportional to the square root of the concentration of the reactant.
D) The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant.
E) The rate of the reaction is directly proportional to the concentration of the reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
6
A gas chromatograph can be used to determine the relative amounts of reactants and products by
A) measuring the changes in pressure as the reaction proceeds.
B) analyzing the intensity of the transmitted light.
C) separating the components of the mixture, which results in an individual peak for each component and the peaks are quantified.
D) measuring the degree of the polarization of light.
E) measuring the change in temperature of the reaction.
A) measuring the changes in pressure as the reaction proceeds.
B) analyzing the intensity of the transmitted light.
C) separating the components of the mixture, which results in an individual peak for each component and the peaks are quantified.
D) measuring the degree of the polarization of light.
E) measuring the change in temperature of the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
7
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A]1,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the overall order of the following reaction,given the rate law? NO(g)+ O3(g)→ NO2(g)+ O2(g)Rate = k[NO][O3]
A) 1st order
B) 2nd order
C) 3rd order
D) 1![<strong>What is the overall order of the following reaction,given the rate law? NO(g)+ O<sub>3</sub>(g)→ NO<sub>2</sub>(g)+ O<sub>2</sub>(g)Rate = k[NO][O<sub>3</sub>]</strong> A) 1st order B) 2nd order C) 3rd order D) 1 order E) 0th order](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c3_d5e7_a96f_277883ace2c8_TB6107_11.jpg) order
order
E) 0th order
A) 1st order
B) 2nd order
C) 3rd order
D) 1
![<strong>What is the overall order of the following reaction,given the rate law? NO(g)+ O<sub>3</sub>(g)→ NO<sub>2</sub>(g)+ O<sub>2</sub>(g)Rate = k[NO][O<sub>3</sub>]</strong> A) 1st order B) 2nd order C) 3rd order D) 1 order E) 0th order](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c3_d5e7_a96f_277883ace2c8_TB6107_11.jpg) order
orderE) 0th order

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
9
Identify the CFC with the longest atmospheric lifetime.
A) CFC-113
B) CFC-12
C) CFC-114
D) CFC-11
E) CFC-115
A) CFC-113
B) CFC-12
C) CFC-114
D) CFC-11
E) CFC-115

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
10
Write a balanced reaction for which the following rate relationships are true. Rate = 
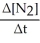 =
= 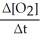 = -
= - 
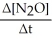
A) N2 + O2 →
N2 + O2 →
 N2O
N2O
B) 2 N2O → 2 N2 + O2
C) N2O → N2 + 2 O2
D) N2O →
N2O →
 N2 + O2
N2 + O2
E) 2 N2 + O2 → 2 N2O

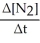 =
= 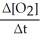 = -
= - 
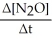
A)
 N2 + O2 →
N2 + O2 →  N2O
N2OB) 2 N2O → 2 N2 + O2
C) N2O → N2 + 2 O2
D)
 N2O →
N2O → N2 + O2
N2 + O2E) 2 N2 + O2 → 2 N2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
11
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A]0,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
12
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A]2,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
13
Determine the rate law and the value of k for the following reaction using the data provided. NO2(g)+ O3(g)→ NO3(g)+ O2(g)[NO2]i (M)[O3]i (M)Initial Rate (M-1s-1)
0)10 0.33 1.42
0)10 0.66 2.84
0)25 0.66 7.10
A) Rate = 1360 M-2.5s-1[NO2]2.5[O3]
B) Rate = 227 M-2.5s-1[NO2][O3]2.5
C) Rate = 43 M-1s-1[NO2][O3]
D) Rate = 430 M-2s-1[NO2]2[O3]
E) Rate = 130 M-2s-1[NO2][O3]2
0)10 0.33 1.42
0)10 0.66 2.84
0)25 0.66 7.10
A) Rate = 1360 M-2.5s-1[NO2]2.5[O3]
B) Rate = 227 M-2.5s-1[NO2][O3]2.5
C) Rate = 43 M-1s-1[NO2][O3]
D) Rate = 430 M-2s-1[NO2]2[O3]
E) Rate = 130 M-2s-1[NO2][O3]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
14
Determine the rate law and the value of k for the following reaction using the data provided. 2 NO(g)+ O2(g)→ 2 NO2(g)[NO]i (M)[O2]i (M)Initial Rate (M-1s-1)
0)030 0.0055 8.55 × 10-3
0)030 0.0110 1.71 × 10-2
0)060 0.0055 3.42 × 10-2
A) Rate = 57 M-1s-1[NO][O2]
B) Rate = 3.8 M-1/2s-1[NO][O2]1/2
C) Rate = 3.1 × 105 M-3s-1[NO]2[O2]2
D) Rate = 1.7 × 103 M-2s-1[NO]2[O2]
E) Rate = 9.4 × 103 M-2s-1[NO][O2]2
0)030 0.0055 8.55 × 10-3
0)030 0.0110 1.71 × 10-2
0)060 0.0055 3.42 × 10-2
A) Rate = 57 M-1s-1[NO][O2]
B) Rate = 3.8 M-1/2s-1[NO][O2]1/2
C) Rate = 3.1 × 105 M-3s-1[NO]2[O2]2
D) Rate = 1.7 × 103 M-2s-1[NO]2[O2]
E) Rate = 9.4 × 103 M-2s-1[NO][O2]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
15
Identify the CFC with the shortest atmospheric lifetime.
A) CFC-113
B) CFC-12
C) CFC-114
D) CFC-11
E) CFC-115
A) CFC-113
B) CFC-12
C) CFC-114
D) CFC-11
E) CFC-115

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
16
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A][B]2,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
17
Write a balanced reaction for which the following rate relationships are true. Rate = - 
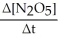 =
= 
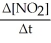 =
= 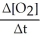
A) 2 N2O5 → 4 NO2 + O2
B) 4 NO2 + O2 → 2 N2O5
C) 2 N2O5 → NO2 + 4 O2
D) NO2 + O2 →
NO2 + O2 →
 N2O5
N2O5
E) N2O5 →
N2O5 →
 NO2 + O2
NO2 + O2

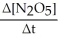 =
= 
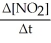 =
= 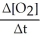
A) 2 N2O5 → 4 NO2 + O2
B) 4 NO2 + O2 → 2 N2O5
C) 2 N2O5 → NO2 + 4 O2
D)
 NO2 + O2 →
NO2 + O2 →  N2O5
N2O5E)
 N2O5 →
N2O5 →  NO2 + O2
NO2 + O2
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
18
Identify the methods used to monitor a reaction as it occurs in the reaction flask.
A) polarimeter
B) spectrometer
C) pressure measurement
D) none of the above
E) all of the above
A) polarimeter
B) spectrometer
C) pressure measurement
D) none of the above
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
19
A rate is equal to 0.0200 M/s.If [A] = 0.100 M and rate = k[A]2[B]2,what is the new rate if the concentration of [A] is increased to 0.200 M?
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s
A) 0.0200 M/s
B) 0.0400 M/s
C) 0.0600 M/s
D) 0.0800 M/s
E) 0.100 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
20
Determine the rate law and the value of k for the following reaction using the data provided. S2O82⁻(aq)+ 3 I⁻(aq)→ 2 SO42⁻(g)+ I3⁻(aq)[S2O82⁻]i (M)[I⁻]i (M)Initial Rate (M-1s-1)
0)30 0.42 4.54
0)44 0.42 6.65
0)44 0.21 3.33
A) Rate = 120 M-2s-1 [S2O82⁻]2[I⁻]
B) Rate = 36 M-1s-1 [S2O82⁻][I⁻]
C) Rate = 86 M-2s-1 [S2O82⁻][I⁻]2
D) Rate = 195 M-3s-1 [S2O82⁻]2[I⁻]2
E) Rate = 23 M-1/2s-1 [S2O82⁻][I⁻]1/2
0)30 0.42 4.54
0)44 0.42 6.65
0)44 0.21 3.33
A) Rate = 120 M-2s-1 [S2O82⁻]2[I⁻]
B) Rate = 36 M-1s-1 [S2O82⁻][I⁻]
C) Rate = 86 M-2s-1 [S2O82⁻][I⁻]2
D) Rate = 195 M-3s-1 [S2O82⁻]2[I⁻]2
E) Rate = 23 M-1/2s-1 [S2O82⁻][I⁻]1/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
21
The second-order decomposition of HI has a rate constant of 1.80 × 10-3 M-1s-1.How much HI remains after 27.3 s if the initial concentration of HI is 4.78 M?
A) 4.55 M
B) 0.258 M
C) 3.87 M
D) 2.20 M
E) 2.39 M
A) 4.55 M
B) 0.258 M
C) 3.87 M
D) 2.20 M
E) 2.39 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
22
The first-order decomposition of N2O5 at 328 K has a rate constant of 1.70 × 10-3 s-1.If the initial concentration of N2O5 is 2.88 M,what is the concentration of N2O5 after 12.5 minutes?
A) 0.124 M
B) 0.805 M
C) 2.82 M
D) 0.355 M
E) 0.174 M
A) 0.124 M
B) 0.805 M
C) 2.82 M
D) 0.355 M
E) 0.174 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
23
The first-order decomposition of N2O at 1000 K has a rate constant of 0.76 s-1.If the initial concentration of N2O is 10.9 M,what is the concentration of N2O after 9.6 s?
A) 7.4 × 10-3 M
B) 1.0 × 10-3 M
C) 1.4 × 10-3 M
D) 3.6 × 10-3 M
E) 8.7 × 10-3 M
A) 7.4 × 10-3 M
B) 1.0 × 10-3 M
C) 1.4 × 10-3 M
D) 3.6 × 10-3 M
E) 8.7 × 10-3 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
24
What data should be plotted to show that experimental concentration data fits a second-order reaction?
A) ln[reactant] vs. time
B) [reactant] vs. time
C) ln(k) vs. 1/T
D) 1/[reactant] vs. time
E) ln(k) vs. Ea
A) ln[reactant] vs. time
B) [reactant] vs. time
C) ln(k) vs. 1/T
D) 1/[reactant] vs. time
E) ln(k) vs. Ea

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
25
The second-order decomposition of NO2 has a rate constant of 0.255 M-1s-1.How much NO2 decomposes in 4.00 s if the initial concentration of NO2 (1.00 L volume)is 1.33 M?
A) 1.8 mol
B) 0.85 mol
C) 0.48 mol
D) 0.77 mol
E) 0.56 mol
A) 1.8 mol
B) 0.85 mol
C) 0.48 mol
D) 0.77 mol
E) 0.56 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
26
The rate constant for the first-order decomposition of N2O is 3.40 s-1.What is the half-life of the decomposition?
A) 0.491 s
B) 0.204 s
C) 0.236 s
D) 0.424 s
E) 0.294 s
A) 0.491 s
B) 0.204 s
C) 0.236 s
D) 0.424 s
E) 0.294 s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following statements is FALSE?
A) The average rate of a reaction decreases during a reaction.
B) It is not possible to determine the rate of a reaction from its balanced equation.
C) The rate of zero-order reactions is not dependent on concentration.
D) The half-life of a first-order reaction is dependent on the initial concentration of reactant.
E) None of the statements is FALSE.
A) The average rate of a reaction decreases during a reaction.
B) It is not possible to determine the rate of a reaction from its balanced equation.
C) The rate of zero-order reactions is not dependent on concentration.
D) The half-life of a first-order reaction is dependent on the initial concentration of reactant.
E) None of the statements is FALSE.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
28
What data should be plotted to show that experimental concentration data fits a first-order reaction?
A) 1/[reactant] vs. time
B) [reactant] vs. time
C) ln[reactant] vs. time
D) ln(k) vs. 1/T
E) ln(k) vs. Ea
A) 1/[reactant] vs. time
B) [reactant] vs. time
C) ln[reactant] vs. time
D) ln(k) vs. 1/T
E) ln(k) vs. Ea

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
29
The first-order decomposition of cyclopropane has a rate constant of 6.7 × 10-4 s-1.If the initial concentration of cyclopropane is 1.33 M,what is the concentration of cyclopropane after 644 s?
A) 0.43 M
B) 0.15 M
C) 0.94 M
D) 0.86 M
E) 0.67 M
A) 0.43 M
B) 0.15 M
C) 0.94 M
D) 0.86 M
E) 0.67 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
30
Identify the rate-determining step.
A) the slowest step
B) the faster step
C) the fast step
D) always the last step
E) always the second step
A) the slowest step
B) the faster step
C) the fast step
D) always the last step
E) always the second step

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
31
The half-life for the second-order decomposition of HI is 15.4 s when the initial concentration of HI is 0.67 M.What is the rate constant for this reaction?
A) 1.0 × 10-2 M-1s-1
B) 4.5 × 10-2 M-1s-1
C) 9.7 × 10-2 M-1s-1
D) 2.2 × 10-2 M-1s-1
E) 3.8 × 10-2 M-1s-1
A) 1.0 × 10-2 M-1s-1
B) 4.5 × 10-2 M-1s-1
C) 9.7 × 10-2 M-1s-1
D) 2.2 × 10-2 M-1s-1
E) 3.8 × 10-2 M-1s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
32
The first-order decay of radon has a half-life of 3.823 days.How many grams of radon decompose after 5.55 days if the sample initially weighs 100.0 grams?
A) 83.4 g
B) 16.6 g
C) 50.0 g
D) 36.6 g
E) 63.4 g
A) 83.4 g
B) 16.6 g
C) 50.0 g
D) 36.6 g
E) 63.4 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements is FALSE?
A) The half-life of a zero-order reaction is dependent on concentration.
B) The half-life of a second-order reaction is not dependent on concentration.
C) The rate of second-order reactions is dependent on concentration.
D) The rate of a first-order reaction is dependent on concentration.
E) None of the statements is FALSE.
A) The half-life of a zero-order reaction is dependent on concentration.
B) The half-life of a second-order reaction is not dependent on concentration.
C) The rate of second-order reactions is dependent on concentration.
D) The rate of a first-order reaction is dependent on concentration.
E) None of the statements is FALSE.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
34
The first-order rearrangement of CH3NC is measured to have a rate constant of 3.61 × 10-15 s-1 at 298 K and a rate constant of 8.66 × 10-7 s-1 at 425 K.Determine the activation energy for this reaction.
A) 160. kJ/mol
B) 240. kJ/mol
C) 417 kJ/mol
D) 127 kJ/mol
E) 338 kJ/mol
A) 160. kJ/mol
B) 240. kJ/mol
C) 417 kJ/mol
D) 127 kJ/mol
E) 338 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
35
The first-order decay of radon has a half-life of 3.823 days.How many grams of radon remain after 7.22 days if the sample initially weighs 250.0 grams?
A) 4.21 g
B) 183 g
C) 54.8 g
D) 76.3 g
E) 67.5 g
A) 4.21 g
B) 183 g
C) 54.8 g
D) 76.3 g
E) 67.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
36
Carbon-14 has a half-life of 5720 years and this is a first-order reaction.If a piece of wood has converted 75% of the carbon-14,then how old is it?
A) 11440 years
B) 2375 years
C) 4750 years
D) 4290 years
E) 1430 years
A) 11440 years
B) 2375 years
C) 4750 years
D) 4290 years
E) 1430 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
37
What data should be plotted to show that experimental concentration data fits a zeroth-order reaction?
A) ln[reactant] vs. time
B) 1/[reactant] vs. time
C) ln(k) vs. 1/T
D) ln(k) vs. Ea
E) [reactant] vs. time
A) ln[reactant] vs. time
B) 1/[reactant] vs. time
C) ln(k) vs. 1/T
D) ln(k) vs. Ea
E) [reactant] vs. time

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
38
Carbon-14 has a half-life of 5720 years and this is a first-order reaction.If a piece of wood has converted 25% of the carbon-14,then how old is it?
A) 11440 years
B) 2375 years
C) 4750 years
D) 4290 years
E) 1430 years
A) 11440 years
B) 2375 years
C) 4750 years
D) 4290 years
E) 1430 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
39
The half-life for the decay of radium is 1620 years.What is the rate constant for this first-order process?
A) 4.28 × 10-4 yr-1
B) 1.12 × 10-4 yr-1
C) 2.33 × 10-4 yr-1
D) 8.91 × 10-4 yr-1
E) 6.17 × 10-4 yr-1
A) 4.28 × 10-4 yr-1
B) 1.12 × 10-4 yr-1
C) 2.33 × 10-4 yr-1
D) 8.91 × 10-4 yr-1
E) 6.17 × 10-4 yr-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
40
For a reaction,what generally happens if the temperature is increased?
A) A decrease in k occurs, which results in a faster rate.
B) A decrease in k occurs, which results in a slower rate.
C) An increase in k occurs, which results in a faster rate.
D) An increase in k occurs, which results in a slower rate.
E) There is no change with k or the rate.
A) A decrease in k occurs, which results in a faster rate.
B) A decrease in k occurs, which results in a slower rate.
C) An increase in k occurs, which results in a faster rate.
D) An increase in k occurs, which results in a slower rate.
E) There is no change with k or the rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
41
Given the following balanced equation,determine the rate of reaction with respect to [O2]. 2 SO2(g)+ O2(g)→ 2 SO3(g)
A) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaae_a96f_8ffd9145643e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaaf_a96f_5dbb06e9b763_TB6107_11.jpg)
B) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c0_a96f_35c7233624da_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c1_a96f_af576e796a4d_TB6107_00.jpg)
C) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c2_a96f_c1725ce30da6_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c3_a96f_b395681ec821_TB6107_00.jpg)
E) It is not possible to determine without more information.
A) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaae_a96f_8ffd9145643e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaaf_a96f_5dbb06e9b763_TB6107_11.jpg)
B) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c0_a96f_35c7233624da_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c1_a96f_af576e796a4d_TB6107_00.jpg)
C) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c2_a96f_c1725ce30da6_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_d1c3_a96f_b395681ec821_TB6107_00.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
42
Identify an heterogeneous catalyst.
A) CFCs with ozone
B) Pd in H2 gas
C) KI dissolved in H2O2
D) H2SO4 with concentrated HCl
E) H3PO4 with an alcohol
A) CFCs with ozone
B) Pd in H2 gas
C) KI dissolved in H2O2
D) H2SO4 with concentrated HCl
E) H3PO4 with an alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
43
If the activation energy for a given compound is found to be 42.0 kJ/mol,with a frequency factor of 8.0 × 1010 s-1,what is the rate constant for this reaction at 298 K?
A) 2.9 × 10-4 s-1
B) 7.4 × 10-4 s-1
C) 1.4 × 109 s-1
D) 4.6 × 105 s-1
E) 3.5 × 103 s-1
A) 2.9 × 10-4 s-1
B) 7.4 × 10-4 s-1
C) 1.4 × 109 s-1
D) 4.6 × 105 s-1
E) 3.5 × 103 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which rate law has a molecularity of three?
A) Rate = k[A]0
B) Rate = k[A][B]2
C) Rate = k[A][B]
D) Rate = k[A]
E) Rate = k[A]2
A) Rate = k[A]0
B) Rate = k[A][B]2
C) Rate = k[A][B]
D) Rate = k[A]
E) Rate = k[A]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
45
Biological catalysts that increase the rates of biochemical reactions are known as
A) substrates.
B) inhibitors.
C) enzymes.
D) binders.
E) trumanettes.
A) substrates.
B) inhibitors.
C) enzymes.
D) binders.
E) trumanettes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
46
If the activation energy for a given compound is found to be 103 kJ/mol,with a frequency factor of 4.0 × 1013 s-1,what is the rate constant for this reaction at 398 K?
A) 1.2 s-1
B) 8.2 s-1
C) 3.9 × 1010 s-1
D) 1.7 × 1010 s-1
E) 2.5 × 107 s-1
A) 1.2 s-1
B) 8.2 s-1
C) 3.9 × 1010 s-1
D) 1.7 × 1010 s-1
E) 2.5 × 107 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
47
In the hydrogenation of double bonds,a catalyst is needed.In the third step,the reactants react to form the product.This step is known as
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
48
A reaction is followed and found to have a rate constant of 3.36 × 104 M-1s-1 at 344 K and a rate constant of 7.69 M-1s-1 at 219 K.Determine the activation energy for this reaction.
A) 23.8 kJ/mol
B) 42.0 kJ/mol
C) 11.5 kJ/mol
D) 12.5 kJ/mol
E) 58.2 kJ/mol
A) 23.8 kJ/mol
B) 42.0 kJ/mol
C) 11.5 kJ/mol
D) 12.5 kJ/mol
E) 58.2 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
49
In the hydrogenation of double bonds,a catalyst is needed.In the first step,the reactants must come into contact with a metal surface.This step is known as
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which rate law has a molecularity of one?
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]2
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
51
In the hydrogenation of double bonds,a catalyst is needed.In the second step,the reactants diffuse on the surface until they approach each other.This step is known as
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
52
In the hydrogenation of double bonds,a catalyst is needed.In the last step,the reactants must escape from the surface into the gas phase.This step is known as
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above
A) adsorption.
B) diffusion.
C) reaction.
D) desorption.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
53
Given the following balanced equation,determine the rate of reaction with respect to [SO2]. 2 SO2(g)+ O2(g)→ 2 SO3(g)
A) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_8398_a96f_6b1901ff7ae5_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_8399_a96f_63fe3d61fb18_TB6107_11.jpg)
B) Rate =![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_839a_a96f_c1b27dacbe6c_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_839b_a96f_27cf14d4585c_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaac_a96f_bd28a47500a3_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaad_a96f_1d797c0718da_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_8398_a96f_6b1901ff7ae5_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_8399_a96f_63fe3d61fb18_TB6107_11.jpg)
B) Rate =
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_839a_a96f_c1b27dacbe6c_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_839b_a96f_27cf14d4585c_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaac_a96f_bd28a47500a3_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>2</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_aaad_a96f_1d797c0718da_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
54
A reaction is found to have an activation energy of 108 kJ/mol.If the rate constant for this reaction is 4.60 × 10-6 s-1 at 275 K,what is the rate constant at 366 K?
A) 12 s-1
B) 1.7 s-1
C) 0.58 s-1
D) 5.4 × 10-5 s-1
E) 1.9 × 10-4 s-1
A) 12 s-1
B) 1.7 s-1
C) 0.58 s-1
D) 5.4 × 10-5 s-1
E) 1.9 × 10-4 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
55
Given the following balanced equation,determine the rate of reaction with respect to [SO3]. 2 SO2(g)+ O2(g)→ 2 SO3(g)
A) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d4_a96f_b18132a7e869_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d5_a96f_d59be89f0a1c_TB6107_11.jpg)
B) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d6_a96f_4b53d3884d81_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe7_a96f_dbc73a869bf5_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe8_a96f_359784e70520_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe9_a96f_17a59da9173f_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d4_a96f_b18132a7e869_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d5_a96f_d59be89f0a1c_TB6107_11.jpg)
B) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c5_f8d6_a96f_4b53d3884d81_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe7_a96f_dbc73a869bf5_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe8_a96f_359784e70520_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [SO<sub>3</sub>]. 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 SO<sub>3</sub>(g)</strong> A) Rate = - B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fe9_a96f_17a59da9173f_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following statements is TRUE?
A) The rate constant does not depend on the activation energy for a reaction where the products are lower in energy than the reactants.
B) A catalyst raises the activation energy of a reaction.
C) Rate constants are temperature dependent.
D) The addition of a homogeneous catalyst does not change the activation energy of a given reaction.
E) None of the above is true.
A) The rate constant does not depend on the activation energy for a reaction where the products are lower in energy than the reactants.
B) A catalyst raises the activation energy of a reaction.
C) Rate constants are temperature dependent.
D) The addition of a homogeneous catalyst does not change the activation energy of a given reaction.
E) None of the above is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which rate law has a molecularity of three?
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]2
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which rate law is unimolecular?
A) Rate = k[A]2
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]3
A) Rate = k[A]2
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which rate law has a molecularity of two?
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]3
A) Rate = k[A]0
B) Rate = k[A][B]
C) Rate = k[A][B][C]
D) Rate = k[A]
E) Rate = k[A]3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
60
A reaction is found to have an activation energy of 38.0 kJ/mol.If the rate constant for this reaction is 1.60 × 102 M-1s-1 at 249 K,what is the rate constant at 436 K?
A) 2.38 × 105 M-1s-1
B) 1.26 × 103 M-1s-1
C) 7.94 × 104 M-1s-1
D) 4.20 × 105 M-1s-1
E) 3.80 × 104 M-1s-1
A) 2.38 × 105 M-1s-1
B) 1.26 × 103 M-1s-1
C) 7.94 × 104 M-1s-1
D) 4.20 × 105 M-1s-1
E) 3.80 × 104 M-1s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
61
What are the units of k in the following rate law? Rate = k[X] [Y]3
A)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df40_a96f_df51fc4777be_TB6107_11.jpg)
B)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df41_a96f_7b5d580b34ad_TB6107_11.jpg)
C) M s2
D)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df42_a96f_5f74055b1adf_TB6107_11.jpg)
E)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df43_a96f_5502b2aeb8a9_TB6107_11.jpg)
A)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df40_a96f_df51fc4777be_TB6107_11.jpg)
B)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df41_a96f_7b5d580b34ad_TB6107_11.jpg)
C) M s2
D)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df42_a96f_5f74055b1adf_TB6107_11.jpg)
E)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup> </sup>[Y]<sup>3</sup></strong> A) B) C) M<sup> </sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_df43_a96f_5502b2aeb8a9_TB6107_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
62
What are the units of k in a zero-order reaction?
A)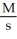
B) M
C)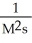
D)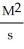
E)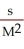
A)
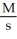
B) M
C)
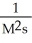
D)
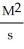
E)
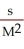

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
63
The decomposition of dinitrogen pentoxide is described by the following chemical equation 2 N2O5(g)→ 4 NO2(g)+ O2(g)
If the rate of appearance of O2 is equal to 3.00 mol/min at a particular moment,what is the rate of disappearance of N2O5 at that moment?
A) 0.750 mol/min
B) 1.50 mol/min
C) 6.00 mol/min
D) 12.0 mol/min
If the rate of appearance of O2 is equal to 3.00 mol/min at a particular moment,what is the rate of disappearance of N2O5 at that moment?
A) 0.750 mol/min
B) 1.50 mol/min
C) 6.00 mol/min
D) 12.0 mol/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
64
What are the units of k in a second-order reaction?
A)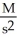
B) M2s2
C)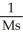
D)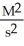
E)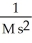
A)
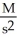
B) M2s2
C)
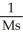
D)
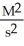
E)
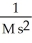

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
65
The decomposition of dinitrogen pentoxide is described by the following chemical equation 2 N2O5(g)→ 4 NO2(g)+ O2(g)
If the rate of disappearance of N2O5 is equal to 1.80 mol/min at a particular moment,what is the rate of appearance of NO2 at that moment?
A) 0.900 mol/min
B) 1.80 mol/min
C) 3.60 mol/min
D) 7.20 mol/min
E) 1.50 mol/min
If the rate of disappearance of N2O5 is equal to 1.80 mol/min at a particular moment,what is the rate of appearance of NO2 at that moment?
A) 0.900 mol/min
B) 1.80 mol/min
C) 3.60 mol/min
D) 7.20 mol/min
E) 1.50 mol/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
66
Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl2(g)→ 2 NOCl(g)
A) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc37_a96f_d9d82e062604_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc38_a96f_c19e054ac9c4_TB6107_11.jpg)
B) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc39_a96f_35af5468e91e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34a_a96f_e361a84f8fae_TB6107_11.jpg)
C) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34b_a96f_6b92f6d94929_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34c_a96f_09769736e75b_TB6107_00.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34d_a96f_a73380e45e4e_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc37_a96f_d9d82e062604_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc38_a96f_c19e054ac9c4_TB6107_11.jpg)
B) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_bc39_a96f_35af5468e91e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34a_a96f_e361a84f8fae_TB6107_11.jpg)
C) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34b_a96f_6b92f6d94929_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34c_a96f_09769736e75b_TB6107_00.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NOCl]. 2 NO(g)+ Cl<sub>2</sub>(g)→ 2 NOCl(g)</strong> A) Rate = - B) Rate = + C) Rate = - D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_e34d_a96f_a73380e45e4e_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
67
Given the following balanced equation,determine the rate of reaction with respect to [O2]. 2 O3(g)→ 3 O2(g)
A) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fea_a96f_f932bedecdd3_TB6107_11.jpg)
B) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fb_a96f_8763fff15054_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fc_a96f_c795acaf2877_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fd_a96f_c51e1cc62b69_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fe_a96f_059d9d65ed24_TB6107_11.jpg)
D) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e0f_a96f_5570447230b0_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_1fea_a96f_f932bedecdd3_TB6107_11.jpg)
B) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fb_a96f_8763fff15054_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fc_a96f_c795acaf2877_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fd_a96f_c51e1cc62b69_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_46fe_a96f_059d9d65ed24_TB6107_11.jpg)
D) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [O<sub>2</sub>]. 2 O<sub>3</sub>(g)→ 3 O<sub>2</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e0f_a96f_5570447230b0_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
68
Given the following balanced equation,determine the rate of reaction with respect to [Cl2].If the rate of Cl2 loss is 4.24 × 10-2 M/s,what is the rate of formation of NOCl? 2 NO(g)+ Cl2(g)→ 2 NOCl(g)
A) 4.24 × 10-2 M/s
B) 2.12 × 10-2 M/s
C) 1.06 × 10-1 M/s
D) 8.48 × 10-2 M/s
E) 1.61 × 10-2 M/s
A) 4.24 × 10-2 M/s
B) 2.12 × 10-2 M/s
C) 1.06 × 10-1 M/s
D) 8.48 × 10-2 M/s
E) 1.61 × 10-2 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
69
What are the units of k in the following rate law? Rate = k[X]2[Y]
A)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_911c_a96f_811f423a1086_TB6107_11.jpg)
B)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82d_a96f_338a6cfc1430_TB6107_11.jpg)
C) M2s2
D)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82e_a96f_a3c7e519ab08_TB6107_11.jpg)
E)![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82f_a96f_69c7ec9cac74_TB6107_11.jpg)
A)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_911c_a96f_811f423a1086_TB6107_11.jpg)
B)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82d_a96f_338a6cfc1430_TB6107_11.jpg)
C) M2s2
D)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82e_a96f_a3c7e519ab08_TB6107_11.jpg)
E)
![<strong>What are the units of k in the following rate law? Rate = k[X]<sup>2</sup>[Y]<sup> </sup></strong> A) B) C) M<sup>2</sup>s<sup>2</sup> D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c8_b82f_a96f_69c7ec9cac74_TB6107_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
70
Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H2(g)+ Cl2(g)→ 2 HCl(g)
A) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a5e_a96f_47513044fc7b_TB6107_00.jpg)
B) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a5f_a96f_fd28f92ca420_TB6107_11.jpg)
C) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a60_a96f_03d8ee864272_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a61_a96f_1da8f8fb5091_TB6107_11.jpg)
D) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3172_a96f_e95badc5ad0e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3173_a96f_dd658cf0dcd5_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a5e_a96f_47513044fc7b_TB6107_00.jpg)
B) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a5f_a96f_fd28f92ca420_TB6107_11.jpg)
C) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a60_a96f_03d8ee864272_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_0a61_a96f_1da8f8fb5091_TB6107_11.jpg)
D) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3172_a96f_e95badc5ad0e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [HCl]. H<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ 2 HCl(g)</strong> A) Rate = + B) Rate = - C) Rate = - D) Rate = + E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3173_a96f_dd658cf0dcd5_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
71
Given the following balanced equation,determine the rate of reaction with respect to [O2].If the rate of formation of O2 is 7.78 × 10-1 M/s,what is the rate of the loss of O3? 2 O3(g)→ 3 O2(g)
A) 0.519 M/s
B) 1.56 M/s
C) 2.34 M/s
D) 1.17 M/s
E) 7.78 M/s
A) 0.519 M/s
B) 1.56 M/s
C) 2.34 M/s
D) 1.17 M/s
E) 7.78 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
72
What are the units of k in the following rate law? Rate = k[X][Y]
A)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0654_a96f_c129f22f6793_TB6107_11.jpg)
B) M s2
C)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0655_a96f_7ba633b95c0a_TB6107_11.jpg)
D)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0656_a96f_2f190c242bf4_TB6107_11.jpg)
E)![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0657_a96f_ebd6489f3de8_TB6107_11.jpg)
A)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0654_a96f_c129f22f6793_TB6107_11.jpg)
B) M s2
C)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0655_a96f_7ba633b95c0a_TB6107_11.jpg)
D)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0656_a96f_2f190c242bf4_TB6107_11.jpg)
E)
![<strong>What are the units of k in the following rate law? Rate = k[X][Y]</strong> A) B) M<sup> </sup>s<sup>2</sup> C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c9_0657_a96f_ebd6489f3de8_TB6107_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
73
Determine the rate law and the value of k for the following reaction using the data provided. 2 N2O5(g)→ 4 NO2(g)+ O2(g)[N2O5]i (M)Initial Rate (M-1s-1)
0)093 4.84 × 10-4
0)084 4.37 × 10-4
0)224 1.16 × 10-3
A) Rate = 5.6 × 10-2 M-1s-1[N2O5]2
B) Rate = 6.0 × 10-1 M-2s-1[N2O5]3
C) Rate = 1.6 × 10-3 M1/2s-1[N2O5]1/2
D) Rate = 1.7 × 10-2 M-1/2s-1[N2O5]3/2
E) Rate = 5.2 × 10⁻3 s-1[N2O5]
0)093 4.84 × 10-4
0)084 4.37 × 10-4
0)224 1.16 × 10-3
A) Rate = 5.6 × 10-2 M-1s-1[N2O5]2
B) Rate = 6.0 × 10-1 M-2s-1[N2O5]3
C) Rate = 1.6 × 10-3 M1/2s-1[N2O5]1/2
D) Rate = 1.7 × 10-2 M-1/2s-1[N2O5]3/2
E) Rate = 5.2 × 10⁻3 s-1[N2O5]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
74
Given the following balanced equation,determine the rate of reaction with respect to [H2]. N2(g)+ 3 H2(g)→ 2 NH3(g)
A) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e10_a96f_15f84884e1fc_TB6107_11.jpg)
B) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e11_a96f_c15b604fd209_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e12_a96f_dd3ae060c77d_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9523_a96f_016fd7f778f9_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9524_a96f_b1f9aeaa5970_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9525_a96f_356b6629738a_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9526_a96f_49212b01fd46_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e10_a96f_15f84884e1fc_TB6107_11.jpg)
B) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e11_a96f_c15b604fd209_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_6e12_a96f_dd3ae060c77d_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9523_a96f_016fd7f778f9_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9524_a96f_b1f9aeaa5970_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9525_a96f_356b6629738a_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [H<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = + C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c6_9526_a96f_49212b01fd46_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
75
The decomposition of dinitrogen pentoxide is described by the following chemical equation 2 N2O5(g)→ 4 NO2(g)+ O2(g)
If the rate of appearance of NO2 is equal to 0.560 mol/min at a particular moment,what is the rate of appearance of O2 at that moment?
A) 0.140 mol/min
B) 0.280 mol/min
C) 1.12 mol/min
D) 2.24 mol/min
If the rate of appearance of NO2 is equal to 0.560 mol/min at a particular moment,what is the rate of appearance of O2 at that moment?
A) 0.140 mol/min
B) 0.280 mol/min
C) 1.12 mol/min
D) 2.24 mol/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
76
Given the following balanced equation,determine the rate of reaction with respect to [NH3]. N2(g)+ 3 H2(g)→ 2 NH3(g)
A) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3174_a96f_e5567d3b6194_TB6107_11.jpg)
B) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5885_a96f_2b00dd2b04aa_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5886_a96f_2181b1ec660e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5887_a96f_9fdbe29a8c33_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5888_a96f_31ce541e9f17_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5889_a96f_8f61a4670837_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_3174_a96f_e5567d3b6194_TB6107_11.jpg)
B) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5885_a96f_2b00dd2b04aa_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5886_a96f_2181b1ec660e_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5887_a96f_9fdbe29a8c33_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5888_a96f_31ce541e9f17_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [NH<sub>3</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_5889_a96f_8f61a4670837_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
77
Given the following balanced equation,determine the rate of reaction with respect to [O2].If the rate of O2 loss is 2.64 × 10-3 M/s,what is the rate of formation of SO3? 2 SO2(g)+ O2(g)→ 2 SO3(g)
A) 2.64 × 10-3 M/s
B) 1.19 × 10-3 M/s
C) 1.32 × 10-3 M/s
D) 6.60 × 10-2 M/s
E) 5.28 × 10-3 M/s
A) 2.64 × 10-3 M/s
B) 1.19 × 10-3 M/s
C) 1.32 × 10-3 M/s
D) 6.60 × 10-2 M/s
E) 5.28 × 10-3 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
78
Given the following balanced equation,determine the rate of reaction with respect to [Cl2].If the rate of disappearance of Cl2 is 4.24 × 10-2 M/s,what is the rate of formation of NO? 2 NO(g)+ Cl2(g)→ 2 NOCl(g)
A) 4.24 × 10-2 M/s
B) 2.12 × 10-2 M/s
C) 1.06 × 10-1 M/s
D) 8.48 × 10-2 M/s
E) 1.61 × 10-2 M/s
A) 4.24 × 10-2 M/s
B) 2.12 × 10-2 M/s
C) 1.06 × 10-1 M/s
D) 8.48 × 10-2 M/s
E) 1.61 × 10-2 M/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
79
Given the following balanced equation,determine the rate of reaction with respect to [N2]. N2(g)+ 3 H2(g)→ 2 NH3(g)
A) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9a_a96f_0bd3d73d95b1_TB6107_11.jpg)
B) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9b_a96f_e9070af07704_TB6107_11.jpg)
C) Rate = +![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9c_a96f_458a6c8495b0_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6ad_a96f_5b71f86696f2_TB6107_11.jpg)
D) Rate = -![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6ae_a96f_f19713b5d3b2_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6af_a96f_01630a7ca4da_TB6107_11.jpg)
E) It is not possible to determine without more information.
A) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9a_a96f_0bd3d73d95b1_TB6107_11.jpg)
B) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9b_a96f_e9070af07704_TB6107_11.jpg)
C) Rate = +
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_7f9c_a96f_458a6c8495b0_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6ad_a96f_5b71f86696f2_TB6107_11.jpg)
D) Rate = -
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6ae_a96f_f19713b5d3b2_TB6107_11.jpg)
![<strong>Given the following balanced equation,determine the rate of reaction with respect to [N<sub>2</sub>]. N<sub>2</sub>(g)+ 3 H<sub>2</sub>(g)→ 2 NH<sub>3</sub>(g)</strong> A) Rate = + B) Rate = - C) Rate = + D) Rate = - E) It is not possible to determine without more information.](https://d2lvgg3v3hfg70.cloudfront.net/TB6107/11ea888e_e2c7_a6af_a96f_01630a7ca4da_TB6107_11.jpg)
E) It is not possible to determine without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck
80
What are the units of k in a first-order reaction?
A)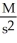
B) M2s
C)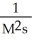
D)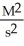
E)
A)
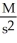
B) M2s
C)
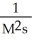
D)
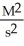
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 155 في هذه المجموعة.
فتح الحزمة
k this deck








