Deck 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/72
العب
ملء الشاشة (f)
Deck 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals
1
For which of the following molecules or ions does the central atom have sp hybridization: N3-,O3,and SF3+?
A) N3- only
B) O3 only
C) SF3+ only
D) O3 and SF3+
E) SF3+and N3-
A) N3- only
B) O3 only
C) SF3+ only
D) O3 and SF3+
E) SF3+and N3-
N3- only
2
How many sigma ( )bonds and pi ( )bonds are in the following molecule? 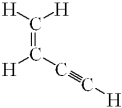
A) seven and three
B) seven and two
C) five and five
D) five and three
E) five and two

A) seven and three
B) seven and two
C) five and five
D) five and three
E) five and two
seven and three
3
The hybridization of the nitrogen atom in NH4+ is _____.
A) sp
B) sp2
C) sp3
D) sp4
E) sp6
A) sp
B) sp2
C) sp3
D) sp4
E) sp6
sp3
4
To form a molecule with a linear electron pair geometry,what set of pure atomic orbitals must be mixed?
A) one s and one p
B) one s and two p
C) one s and three p
D) two s and one p
E) zero s and three p
A) one s and one p
B) one s and two p
C) one s and three p
D) two s and one p
E) zero s and three p

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the hybridization of the central nitrogen atom in N2O?
A) sp
B) sp2
C) sp3
D) none
A) sp
B) sp2
C) sp3
D) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the maximum number of hybridized orbitals that can be formed by a fluorine atom?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following does contain at least one pi bond?
A) HCN
B) C2H6
C) CO
D) C2H2
E) All of them have one or more pi bonds.
A) HCN
B) C2H6
C) CO
D) C2H2
E) All of them have one or more pi bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following statements concerning valence bond (VB)theory is/are CORRECT?
1)VB theory can describe molecular bonding in excited states.
2)VB theory assumes that electrons are localized between pairs of atoms.
3)VB theory predicts localized lone pairs of electrons.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)VB theory can describe molecular bonding in excited states.
2)VB theory assumes that electrons are localized between pairs of atoms.
3)VB theory predicts localized lone pairs of electrons.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
9
How many sigma ( )bonds and pi ( )bonds are in carbon monoxide?
A) three ,zero
B) two ,one
C) two ,two
D) one ,two
E) one ,one
A) three ,zero
B) two ,one
C) two ,two
D) one ,two
E) one ,one

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many sigma and pi bonds are in the molecule pictured below? 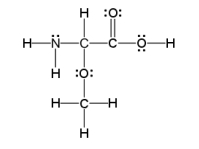
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds

A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the hybridization of each carbon atom in benzene,C6H6? Benzene contains a six-member carbon ring.
A) sp
B) sp2
C) sp3
D) sp4
E) sp5
A) sp
B) sp2
C) sp3
D) sp4
E) sp5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the molecular geometry around a central atom that is sp3 hybridized and has one lone pair of electrons?
A) bent
B) linear
C) trigonal-planar
D) trigonal-pyramidal
E) tetrahedral
A) bent
B) linear
C) trigonal-planar
D) trigonal-pyramidal
E) tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the hybridization of the central atom in a molecule with a trigonal-planar molecular geometry?
A) sp
B) sp2
C) sp3
D) sp4
E) none
A) sp
B) sp2
C) sp3
D) sp4
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following statements is/are CORRECT according to valence bond theory?
1)Elelctron density in a pi bond is greatest along the axis of the bond.
2)The overlap of two adjacent p orbitals can result in a sigma bond or a pi bond,depending on the p-orbital orientations.
3)Bonds are formed in molecules through the overlap of valence electron atomic orbitals.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)Elelctron density in a pi bond is greatest along the axis of the bond.
2)The overlap of two adjacent p orbitals can result in a sigma bond or a pi bond,depending on the p-orbital orientations.
3)Bonds are formed in molecules through the overlap of valence electron atomic orbitals.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the hybridization of the sulfur atom in SO42-?
A) sp
B) sp2
C) sp3
D) none
A) sp
B) sp2
C) sp3
D) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
16
For which of the following molecules and ions does the central atom have sp2 hybridization? (The central atom is listed first in each formula below.)
A) SO42-
B) CO2
C) NOBr
D) SBr2
E) N2O
A) SO42-
B) CO2
C) NOBr
D) SBr2
E) N2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the hybridization of the sulfur atom in SCl2?
A) sp
B) sp2
C) sp3
D) none
A) sp
B) sp2
C) sp3
D) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following statements concerning molecular orbital (MO)bond theory is/are CORRECT?
1)MO theory can describe molecular bonding in excited states.
2)Molecular orbitals are obtained from the combination of atomic orbitals.
3)MO theory predicts that electrons are delocalized over the molecule through molecular orbitals.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)MO theory can describe molecular bonding in excited states.
2)Molecular orbitals are obtained from the combination of atomic orbitals.
3)MO theory predicts that electrons are delocalized over the molecule through molecular orbitals.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following statements concerning hybrid orbitals is/are CORRECT?
1)The number of hybrid orbitals equals the number of atomic orbitals that are used to create the hybrids.
2)When atomic orbitals are hybridized,the s orbital and at least one p orbital are always hybridized.
3)To create tetrahedral structures,the s orbital and all three p orbitals must be hybridized.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)The number of hybrid orbitals equals the number of atomic orbitals that are used to create the hybrids.
2)When atomic orbitals are hybridized,the s orbital and at least one p orbital are always hybridized.
3)To create tetrahedral structures,the s orbital and all three p orbitals must be hybridized.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
20
For which of the following molecules does the carbon atom have sp3 hybridization?
A) Cl2CO
B) CO
C) CS2
D) CH2Cl2
E) HCN
A) Cl2CO
B) CO
C) CS2
D) CH2Cl2
E) HCN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
21
All of the following statements concerning molecular orbital (MO)theory are correct EXCEPT
A) the Pauli exclusion principle is obeyed.
B) Hund's rule is obeyed.
C) electrons are assigned to orbitals of successively higher energy.
D) a bonding molecular orbital is lower in energy than its parent atomic orbitals.
E) the combination of two atomic orbitals creates only one molecular orbital.
A) the Pauli exclusion principle is obeyed.
B) Hund's rule is obeyed.
C) electrons are assigned to orbitals of successively higher energy.
D) a bonding molecular orbital is lower in energy than its parent atomic orbitals.
E) the combination of two atomic orbitals creates only one molecular orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
22
Ammonia reacts with oxygen and water to produce nitric acid.What change in hybridization of the nitrogen atom occurs in this reaction?
A) sp3 to sp2
B) sp3 to sp
C) sp2 to sp3
D) sp2 to sp
E) no change
A) sp3 to sp2
B) sp3 to sp
C) sp2 to sp3
D) sp2 to sp
E) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following concerning and bonds is/are correct?
1)Sigma bonds may only be formed from unhybridized orbitals.
2)Pi bonds are formed from unhybridized p orbitals.
3)A pi bond has an electron distribution above and below the bond axis.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
1)Sigma bonds may only be formed from unhybridized orbitals.
2)Pi bonds are formed from unhybridized p orbitals.
3)A pi bond has an electron distribution above and below the bond axis.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
24
Upon combustion,ethene (C2H4)is converted to carbon dioxide and water.What change in the hybridization of carbon occurs in this reaction?
A) sp to sp2
B) sp2 to sp3
C) sp3 to sp
D) sp2 to sp
E) sp3 to sp2
A) sp to sp2
B) sp2 to sp3
C) sp3 to sp
D) sp2 to sp
E) sp3 to sp2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
25
For which of the following compounds is it possible for cis and trans isomers to exist? 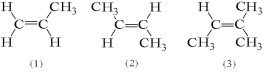
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the underlined atoms (C1,C2,N,and O)are sp hybridized? 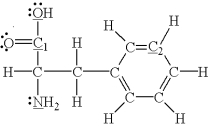
A) C1 and C2
B) C1,C2,N,and O
C) N and O
D) C1,C2,and N
E) none

A) C1 and C2
B) C1,C2,N,and O
C) N and O
D) C1,C2,and N
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the hybridization of an atom that has 1 bond,0 bonds,and 3 lone pairs?
A) sp
B) sp2
C) sp3
D) sp4
E) none
A) sp
B) sp2
C) sp3
D) sp4
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
28
Dichloromethane,CH2Cl2,is a common organic solvent.Which of the following statements concerning dichloromethane is/are CORRECT?
1)CH2Cl2 has two isomers.For one isomer of CH2Cl2,the chlorine atoms are adjacent to each other and the molecule is polar.
2)CH2Cl2 has two isomers.For one isomer of CH2Cl2,the chlorine atoms are on opposites sides of the carbon atom and the molecule is nonpolar.
3)The hybridization of the central carbon atom is sp3.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)CH2Cl2 has two isomers.For one isomer of CH2Cl2,the chlorine atoms are adjacent to each other and the molecule is polar.
2)CH2Cl2 has two isomers.For one isomer of CH2Cl2,the chlorine atoms are on opposites sides of the carbon atom and the molecule is nonpolar.
3)The hybridization of the central carbon atom is sp3.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the hybridization of the nitrogen atom? 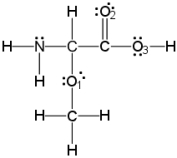
A) sp
B) sp2
C) sp3
D) sp4
E) none

A) sp
B) sp2
C) sp3
D) sp4
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following characteristics apply to NCl3?
1)polar bonds
2)nonpolar molecule
3)triangular-planar molecular shape
4)sp2 hybridized N atom
A) 1 only
B) 1 and 2
C) 3 and 4
D) 1,2,and 3
E) 1,2,3,and 4
1)polar bonds
2)nonpolar molecule
3)triangular-planar molecular shape
4)sp2 hybridized N atom
A) 1 only
B) 1 and 2
C) 3 and 4
D) 1,2,and 3
E) 1,2,3,and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
31
Nitric acid,HNO3,dissociates in water to form nitrate ions and hydronium ions.What change in hybridization of the nitrogen atom occurs in this dissociation?
A) sp2 to sp3
B) sp2 to sp
C) sp3 to sp
D) sp to sp3
E) no change
A) sp2 to sp3
B) sp2 to sp
C) sp3 to sp
D) sp to sp3
E) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following hybridized atoms is not possible?
A) an sp hybridized nitrogen atom
B) an sp2 hybridized oxygen atom
C) an sp3 hybridized boron atom
D) an sp3 hybridized phosphorus atom
E) an sp3 hybridized hydrogen atom
A) an sp hybridized nitrogen atom
B) an sp2 hybridized oxygen atom
C) an sp3 hybridized boron atom
D) an sp3 hybridized phosphorus atom
E) an sp3 hybridized hydrogen atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the molecular geometry around a central atom that is sp2 hybridized,has three sigma bonds,and one pi bond?
A) trigonal-planar
B) trigonal-pyramidal
C) bent
D) T-shaped
E) tetrahedral
A) trigonal-planar
B) trigonal-pyramidal
C) bent
D) T-shaped
E) tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
34
Atomic orbitals combine most effectively to form molecular orbitals when
A) electrons in the orbitals have no spins.
B) electrons in the orbitals have the same spin.
C) the atoms have an equal number of valence electrons.
D) the atomic orbitals have similar energies.
E) only d-orbitals are used in bonding.
A) electrons in the orbitals have no spins.
B) electrons in the orbitals have the same spin.
C) the atoms have an equal number of valence electrons.
D) the atomic orbitals have similar energies.
E) only d-orbitals are used in bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following concerning and bonds is/are correct?
1)A sigma bond may be formed from the sideways overlap of two parallel p orbitals.
2)No more than two pi bonds are possible between adjacent carbon atoms.
3)The considerable energy required to rotate pi bonded atoms is the primary reason for geometrical isomerism in some pi bonded molecules.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
1)A sigma bond may be formed from the sideways overlap of two parallel p orbitals.
2)No more than two pi bonds are possible between adjacent carbon atoms.
3)The considerable energy required to rotate pi bonded atoms is the primary reason for geometrical isomerism in some pi bonded molecules.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the labeled carbon atoms (C1-C4)is/are sp hybridized? 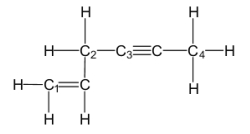
A) C1
B) C1 and C2
C) C3
D) C2 and C4
E) C1 and C3

A) C1
B) C1 and C2
C) C3
D) C2 and C4
E) C1 and C3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
37
When an atom in a molecule or ion is described as sp3 hybridized,its electron pair geometry is
A) linear
B) triangular planar
C) triangular pyramidal
D) bent
E) tetrahedral
A) linear
B) triangular planar
C) triangular pyramidal
D) bent
E) tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
38
A molecular orbital that decreases the electron density between two nuclei is said to be ____.
A)hybridized
B)bonding
B)antibonding
D)pi-bonding
E)nonpolar
A)hybridized
B)bonding
B)antibonding
D)pi-bonding
E)nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the molecular geometry around an atom that has 2 bonds,0 bonds,and 1 lone pair of electrons?
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
40
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
A) B2+
B) H2
C) F2+
D) N2+
E) O22-

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
A) B2+
B) H2
C) F2+
D) N2+
E) O22-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
41
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.Consider the molecules B2,C2,N2 and F2.Which two molecules have the same bond order?
A) B2 and C2
B) B2 and F2
C) C2 and N2
D) C2 and F2
E) N2 and F2

-Refer to Diagram 9-1.Consider the molecules B2,C2,N2 and F2.Which two molecules have the same bond order?
A) B2 and C2
B) B2 and F2
C) C2 and N2
D) C2 and F2
E) N2 and F2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
42
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have only unpaired electron?
A) B2
B) O22-
C) C2
D) O2
E) N2+

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have only unpaired electron?
A) B2
B) O22-
C) C2
D) O2
E) N2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which molecule will have the following valence molecular orbital level energy diagram?
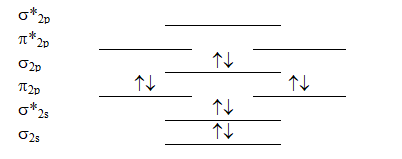
A) Li2
B) Be2
C) B2
D) C2
E) N2

A) Li2
B) Be2
C) B2
D) C2
E) N2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
44
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to diagram 9-1.Identify the molecule with the shortest bond length.
A) O2
B) C2
C) B2
D) F2
E) N2

-Refer to diagram 9-1.Identify the molecule with the shortest bond length.
A) O2
B) C2
C) B2
D) F2
E) N2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
45
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species is paramagnetic.
A) NO+
B) CO
C) CN-
D) OF-
E) NO

-Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species is paramagnetic.
A) NO+
B) CO
C) CN-
D) OF-
E) NO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the bond order for a molecule with 6 electrons in bonding molecular orbitals and 6 electrons in antibonding molecular orbitals?
A) 0
B) 0.5
C) 1
D) 1.5
E) 2
A) 0
B) 0.5
C) 1
D) 1.5
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
47
What does the following figure represent? 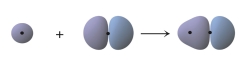
A) the overlap of two 1s orbitals to form a bond
B) the overlap of two 2p orbitals to form a bond
C) the overlap of two 2p orbitals to form a bond
D) the overlap of a 1s orbital and a 2p orbital to form a bond
E) the overlap of a 1s orbital and a 2p orbital to form a bond
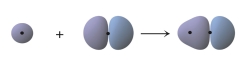
A) the overlap of two 1s orbitals to form a bond
B) the overlap of two 2p orbitals to form a bond
C) the overlap of two 2p orbitals to form a bond
D) the overlap of a 1s orbital and a 2p orbital to form a bond
E) the overlap of a 1s orbital and a 2p orbital to form a bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
48
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. ![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.Assume that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule.What is the molecular orbital configuration of CO?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>2</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>4</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>6</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>3</sup> ( \sigma <sub>2p</sub>)<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.Assume that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule.What is the molecular orbital configuration of CO?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)2 ( *2p)2
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)4
D) [core electrons] ( 2s)2 ( *2s)2 ( 2p)6
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)3 ( 2p)3
![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.Assume that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule.What is the molecular orbital configuration of CO?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>2</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>4</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>6</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>3</sup> ( \sigma <sub>2p</sub>)<sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.Assume that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule.What is the molecular orbital configuration of CO?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)2 ( *2p)2
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)4
D) [core electrons] ( 2s)2 ( *2s)2 ( 2p)6
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)3 ( 2p)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
49
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,what is the bond order of C2+?
A) 1
B) 1.5
C) 2
D) 2.5
E) 3

-Refer to Diagram 9-1.According to molecular orbital theory,what is the bond order of C2+?
A) 1
B) 1.5
C) 2
D) 2.5
E) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
50
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. ![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of N<sub>2</sub><sup>2+</sup>?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma sub>2p</sub>)<sup>2</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>4</sup> ( \sigma *<sub>2s</sub>)<sup>4</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( sub>2p)2
D) [core electrons] ( 2s)4 ( *2s)4
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)4
![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of N<sub>2</sub><sup>2+</sup>?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma sub>2p</sub>)<sup>2</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>4</sup> ( \sigma *<sub>2s</sub>)<sup>4</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( sub>2p)2
D) [core electrons] ( 2s)4 ( *2s)4
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
51
If 10 orbitals on one atom overlap 10 orbitals on a second atom,how many molecular orbitals will form?
A) 1
B) 2
C) 10
D) 20
E) none of these
A) 1
B) 2
C) 10
D) 20
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
52
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to diagram 9-1.Identify the molecule or ion with the longest bond length.
A) O2
B) O2+
C) O2-
D) O22-
E) O22+

-Refer to diagram 9-1.Identify the molecule or ion with the longest bond length.
A) O2
B) O2+
C) O2-
D) O22-
E) O22+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
53
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. ![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of F<sub>2</sub>?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>2</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \pi *<sub>2p</sub>)<sup>4</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>6</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)2 ( *2p)2
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( *2p)4
D) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)6
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)4
![<strong>Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of F<sub>2</sub>?</strong> A) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>2</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>2</sup> C) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \pi *<sub>2p</sub>)<sup>4</sup> D) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \sigma *<sub>2p</sub>)<sup>6</sup> E) [core electrons] ( \sigma <sub>2s</sub>)<sup>2</sup> ( \sigma *<sub>2s</sub>)<sup>2</sup> ( \pi <sub>2p</sub>)<sup>4</sup> ( \sigma <sub>2p</sub>)<sup>2</sup> ( \pi *<sub>2p</sub>)<sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg)
-Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
A) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)2
B) [core electrons] ( 2s)2 ( *2s)2 ( 2p)2 ( 2p)2 ( *2p)2
C) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( *2p)4
D) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)6
E) [core electrons] ( 2s)2 ( *2s)2 ( 2p)4 ( 2p)2 ( *2p)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
54
The electron configuration of a particular diatomic species is:
[core electrons]( 2s)2( *2s)2( 2p)2( 2p)2( *2p)0
What is the bond order for this species?
A) 1
B) 1.5
C) 2
D) 2.5
E) 3
[core electrons]( 2s)2( *2s)2( 2p)2( 2p)2( *2p)0
What is the bond order for this species?
A) 1
B) 1.5
C) 2
D) 2.5
E) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
55
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following lists ranks the fluorine species in terms of increasing bond order?
A) F22+ < F22- < F2
B) F22- < F2 < F22+
C) F2 < F22+ < F22-
D) F2 < F22- < F22+
E) F22+ < F2 < F22-

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following lists ranks the fluorine species in terms of increasing bond order?
A) F22+ < F22- < F2
B) F22- < F2 < F22+
C) F2 < F22+ < F22-
D) F2 < F22- < F22+
E) F22+ < F2 < F22-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
56
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species has the highest bond order.
A) NO-
B) OF-
C) C2
D) O22-
E) NO+

-Refer to Diagram 9-1.Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a heteronuclear diatomic molecule,determine which of the following species has the highest bond order.
A) NO-
B) OF-
C) C2
D) O22-
E) NO+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
57
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,what is the bond order of Li22+?
A) 0
B) 0.5
C) 1
D) 1.5
E) 2

-Refer to Diagram 9-1.According to molecular orbital theory,what is the bond order of Li22+?
A) 0
B) 0.5
C) 1
D) 1.5
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
58
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
A) B2+
B) Ne22+
C) C2+
D) O22+
E) B2

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
A) B2+
B) Ne22+
C) C2+
D) O22+
E) B2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
59
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be diamagnetic?
A) He2+
B) F2-
C) Li2+
D) F2
E) C2+

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be diamagnetic?
A) He2+
B) F2-
C) Li2+
D) F2
E) C2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
60
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
A) O22-
B) Li2
C) O2-
D) C2
E) F2

-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
A) O22-
B) Li2
C) O2-
D) C2
E) F2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
61
The following valence molecular orbital energy level diagram is appropriate for which one of the listed species?
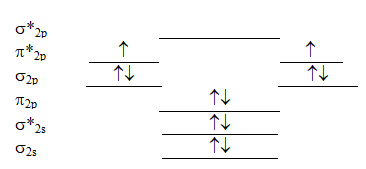
A) B22+
B) C22+
C) N22+
D) O22+
E) F22+

A) B22+
B) C22+
C) N22+
D) O22+
E) F22+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
62
In the NO2- ion,each atom can be viewed as sp2 hybridized.Thus,each atom has one remaining unhybridized p orbital.How many 2p molecular orbitals (including both bonding and antibonding orbitals)are formed using the unhybridized p orbitals?
A) 1
B) 3
C) 4
D) 6
E) 12
A) 1
B) 3
C) 4
D) 6
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
63
SF3+,has a tetrahedral electron-pair geometry and a trigonal-pyramidal molecular geometry.The hybridization of the central sulfur atom is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
64
In valence bond theory,each sigma bond in CH4 is formed from the overlap of a hydrogen atom's 1s orbital with a(n)________ hybridized orbital on the carbon atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
65
The hybridization of the carbon atom in CF3+ is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
66
In HF2- the hydrogen is shared between the two fluorine atoms.Which theory,valence bond or molecule orbital,correctly accounts for this three-center bonding?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
67
In molecular orbital theory,the bond order is defined as 1/2 (the number of electrons in ________ orbitals minus the number of electrons in antibonding orbitals).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
68
Draw a Lewis structure of xenon trioxide.What is the hybridization of the xenon atom in this molecule?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which molecule will have the following valence molecular orbital energy level diagram?
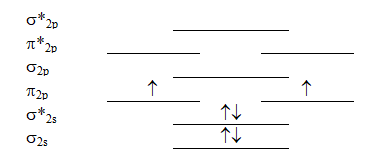
A) Be2
B) B2
C) C2
D) N2
E) O2

A) Be2
B) B2
C) C2
D) N2
E) O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which diatomic molecule or ion has valence electron molecular orbital configuration provided below?
[core electrons]( 2s)2( *2s)2( 2p)2( 2p)4( *2p)1
A) C2
B) O2+
C) O2+.
D) Ne2
E) H2
[core electrons]( 2s)2( *2s)2( 2p)2( 2p)4( *2p)1
A) C2
B) O2+
C) O2+.
D) Ne2
E) H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
71
Benzene,C6H6,consists of a six member ring of sp2 hybridized carbon atoms.Each carbon atom has one unhybridized p orbital.How many 2p bonding,antibonding,and nonbonding molecular orbitals exist for benzene?
A) Three 2p molecular orbitals exist; two bonding and one antibonding.
B) Three 2p molecular orbitals exist; one bonding,one antibonding,and one nonbonding.
C) Six 2p molecular orbitals exist; three bonding and three antibonding.
D) Six 2p molecular orbitals exist; two bonding,two nonbonding,and two antibonding.
E) Twelve 2p molecular orbitals exist; six bonding and six antibonding.
A) Three 2p molecular orbitals exist; two bonding and one antibonding.
B) Three 2p molecular orbitals exist; one bonding,one antibonding,and one nonbonding.
C) Six 2p molecular orbitals exist; three bonding and three antibonding.
D) Six 2p molecular orbitals exist; two bonding,two nonbonding,and two antibonding.
E) Twelve 2p molecular orbitals exist; six bonding and six antibonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which theory,valence bond or molecule orbital,correctly predicts the existence of paramagnetic molecules?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 72 في هذه المجموعة.
فتح الحزمة
k this deck








