Deck 16: Principles of Reactivity: Chemical Equilibria
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/82
العب
ملء الشاشة (f)
Deck 16: Principles of Reactivity: Chemical Equilibria
1
When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below.CO(g)+ 3H2(g) 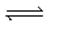 CH4(g)+ H2O(g)
CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C?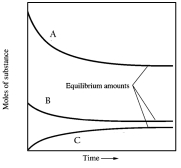
A) hydrogen
B) carbon monoxide
C) either methane or water
D) either hydrogen or carbon monoxide
E) not enough information to decide
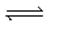 CH4(g)+ H2O(g)
CH4(g)+ H2O(g)In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve C?

A) hydrogen
B) carbon monoxide
C) either methane or water
D) either hydrogen or carbon monoxide
E) not enough information to decide
either methane or water
2
Ozone is formed from oxygen.3 O2(g)  2 O3(g)
2 O3(g)
Calculate the value of Kp,given that Kc = 2.5 10-29 at 298 K.(R = 0.08206 L.atm/mol.K)
A) 1.0 10-30
B) 2.1 10-30
C) 2.5 10-29
D) 3.3 10-28
E) 6.1 10-28
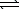 2 O3(g)
2 O3(g)Calculate the value of Kp,given that Kc = 2.5 10-29 at 298 K.(R = 0.08206 L.atm/mol.K)
A) 1.0 10-30
B) 2.1 10-30
C) 2.5 10-29
D) 3.3 10-28
E) 6.1 10-28
1.0 10-30
3
Write the expression for K for the reaction below.
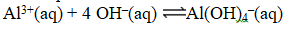
A)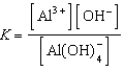
B)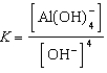
C)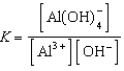
D)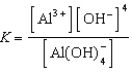
E)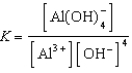
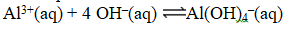
A)
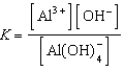
B)
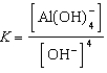
C)
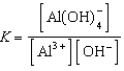
D)
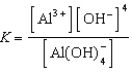
E)
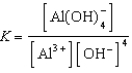
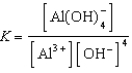
4
Which of the following statements is/are CORRECT?
1)For a chemical system,if the reaction quotient (Q)is greater than K,reactant must be converted to products to reach equilibrium.
2)For a chemical system at equilibrium,the forward and reverse rates of reaction are equal.
3)For a chemical system at equilibrium,the concentrations of products divided by the concentrations of reactants equals one.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)For a chemical system,if the reaction quotient (Q)is greater than K,reactant must be converted to products to reach equilibrium.
2)For a chemical system at equilibrium,the forward and reverse rates of reaction are equal.
3)For a chemical system at equilibrium,the concentrations of products divided by the concentrations of reactants equals one.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
5
For the reaction NO(g)+ ½ O2(g)  NO2(g)at 750°C,what is the relationship between Kc and Kp?
NO2(g)at 750°C,what is the relationship between Kc and Kp?
A) Kc = Kp
B) Kc = Kp (RT)-½
C) Kc = Kp = 1.0
D) Kc = Kp (RT)¾
E) Kc = Kp (RT)½
 NO2(g)at 750°C,what is the relationship between Kc and Kp?
NO2(g)at 750°C,what is the relationship between Kc and Kp?A) Kc = Kp
B) Kc = Kp (RT)-½
C) Kc = Kp = 1.0
D) Kc = Kp (RT)¾
E) Kc = Kp (RT)½

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
6
Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 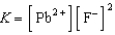
A) PbF2(aq) Pb(s)+ F2(aq)
Pb(s)+ F2(aq)
B) PbF2(s) Pb2+(aq)+ 2 F-(aq)
Pb2+(aq)+ 2 F-(aq)
C) Pb2+(aq)+ 2 F-(aq) PbF2(s)
PbF2(s)
D) Pb(s)+ F2(aq) PbF2(aq)
PbF2(aq)
E) PbF+(aq)+ F-(aq) PbF2(aq)
PbF2(aq)
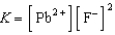
A) PbF2(aq)
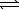 Pb(s)+ F2(aq)
Pb(s)+ F2(aq)B) PbF2(s)
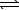 Pb2+(aq)+ 2 F-(aq)
Pb2+(aq)+ 2 F-(aq)C) Pb2+(aq)+ 2 F-(aq)
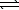 PbF2(s)
PbF2(s)D) Pb(s)+ F2(aq)
 PbF2(aq)
PbF2(aq)E) PbF+(aq)+ F-(aq)
 PbF2(aq)
PbF2(aq)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
7
For which one of the following reactions does Kp equal Kc?
A) 2 CO2(g) 2 CO(g)+ O2(g)
2 CO(g)+ O2(g)
B) CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(g)
CO2(g)+ 2 H2O(g)
C) C(s)+ H2O(g)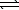 H2(g)+ CO(g)
H2(g)+ CO(g)
D) NH3(g)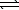 3/2 H2(g)+ 1/2 N2(g)
3/2 H2(g)+ 1/2 N2(g)
E) 2 O3(g)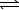 3 O2(g)
3 O2(g)
A) 2 CO2(g)
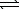 2 CO(g)+ O2(g)
2 CO(g)+ O2(g)B) CH4(g)+ 2 O2(g)
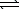 CO2(g)+ 2 H2O(g)
CO2(g)+ 2 H2O(g)C) C(s)+ H2O(g)
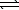 H2(g)+ CO(g)
H2(g)+ CO(g)D) NH3(g)
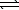 3/2 H2(g)+ 1/2 N2(g)
3/2 H2(g)+ 1/2 N2(g)E) 2 O3(g)
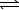 3 O2(g)
3 O2(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the Kc expression for the following equilibrium?
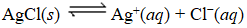
A)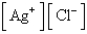
B)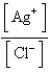
C)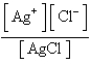
D)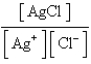
E)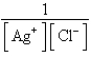
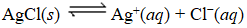
A)
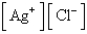
B)

C)
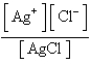
D)
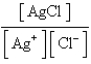
E)
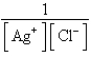

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
9
Write the expression for Kp for the reaction below.
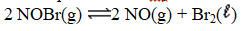
A)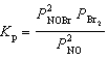
B)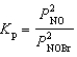
C)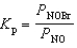
D)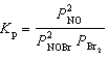
E)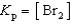
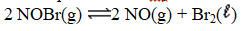
A)
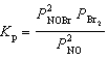
B)
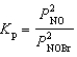
C)
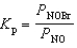
D)
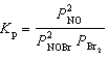
E)
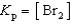

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which expression correctly describes the equilibrium constant Kc for the following reaction?

A)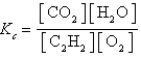
B)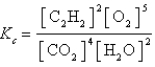
C)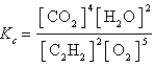
D)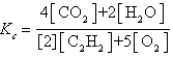
E)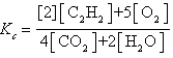

A)
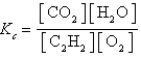
B)
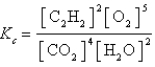
C)
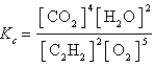
D)
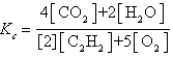
E)
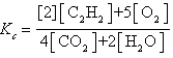

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
11
What balanced equation is the following equilibrium expression derived from? 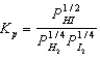
A)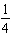 H2(g)+
H2(g)+ 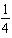 I2(g)
I2(g) 
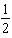 HI(g)
HI(g)
B)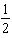 HI(g)
HI(g) 
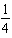 H2(g)+
H2(g)+ 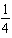 I2(g)
I2(g)
C)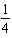 H2(aq)+
H2(aq)+ 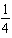 I2(aq)
I2(aq) 
 HI(aq)
HI(aq)
D)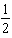 HI(aq)
HI(aq) 
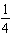 H2(aq)+
H2(aq)+ 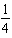 I2(aq)
I2(aq)
E) 2HI(g) H2(g)+ I2(g)
H2(g)+ I2(g)
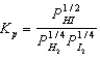
A)
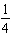 H2(g)+
H2(g)+ 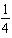 I2(g)
I2(g) 
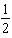 HI(g)
HI(g)B)
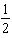 HI(g)
HI(g) 
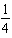 H2(g)+
H2(g)+ 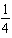 I2(g)
I2(g)C)
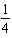 H2(aq)+
H2(aq)+ 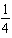 I2(aq)
I2(aq) 
 HI(aq)
HI(aq)D)
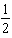 HI(aq)
HI(aq) 
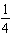 H2(aq)+
H2(aq)+ 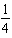 I2(aq)
I2(aq)E) 2HI(g)
 H2(g)+ I2(g)
H2(g)+ I2(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following statements is/are CORRECT?
1)Product concentrations appear in the numerator of an equilibrium constant expression.
2)A reaction favors the formation of products if K >> 1.
3)Stoichiometric coefficients are used as exponents in an equilibrium constant expression.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)Product concentrations appear in the numerator of an equilibrium constant expression.
2)A reaction favors the formation of products if K >> 1.
3)Stoichiometric coefficients are used as exponents in an equilibrium constant expression.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
13
For which of the following equilibria does Kc = Kp?
A) N2(g)+ 3H2(g) 2NH3(g)
2NH3(g)
B) CO(g)+ H2O(g) CO2(g)+ H2(g)
CO2(g)+ H2(g)
C) CO(g)+ 3H2(g) CH4(g)+ H2O(g)
CH4(g)+ H2O(g)
D) CaO(s)+ CO2(g) CaCO3(s)
CaCO3(s)
E) HBr(g) ½H2(g)+ ½Br2(l)
½H2(g)+ ½Br2(l)
A) N2(g)+ 3H2(g)
 2NH3(g)
2NH3(g)B) CO(g)+ H2O(g)
 CO2(g)+ H2(g)
CO2(g)+ H2(g)C) CO(g)+ 3H2(g)
 CH4(g)+ H2O(g)
CH4(g)+ H2O(g)D) CaO(s)+ CO2(g)
 CaCO3(s)
CaCO3(s)E) HBr(g)
 ½H2(g)+ ½Br2(l)
½H2(g)+ ½Br2(l)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
14
Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 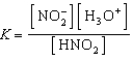
A) HNO2(aq)+ H2O(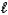 )
) 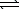 NO2-(aq)+ H3O+(aq)
NO2-(aq)+ H3O+(aq)
B) NO2-(aq)+ H3O+(aq) HNO2(aq)+ H2O(
HNO2(aq)+ H2O(  )
)
C) NO2-(aq)+ H3O+(aq) HNO2(aq)
HNO2(aq)
D) H+(aq)+ OH-(aq) H2O(
H2O( 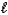 )
)
E) HNO2(aq)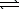 NO2-(aq)+ H3O+(aq)
NO2-(aq)+ H3O+(aq)
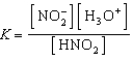
A) HNO2(aq)+ H2O(
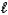 )
)  NO2-(aq)+ H3O+(aq)
NO2-(aq)+ H3O+(aq)B) NO2-(aq)+ H3O+(aq)
 HNO2(aq)+ H2O(
HNO2(aq)+ H2O(  )
)C) NO2-(aq)+ H3O+(aq)
 HNO2(aq)
HNO2(aq)D) H+(aq)+ OH-(aq)
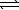 H2O(
H2O( 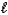 )
)E) HNO2(aq)
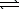 NO2-(aq)+ H3O+(aq)
NO2-(aq)+ H3O+(aq)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
15
Given the following chemical equilibrium,
COCl2(g)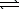
CO(g)+ Cl2(g)
Calculate the value of Kc,given that Kp = 6.5 1011 at 298 K.(R = 0.08206 L.atm/mol.K)
A) 1.5 10-12
B) 3.8 10-11
C) 1.1 109
D) 2.7 1010
E) 1.6 1013
COCl2(g)
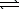
CO(g)+ Cl2(g)
Calculate the value of Kc,given that Kp = 6.5 1011 at 298 K.(R = 0.08206 L.atm/mol.K)
A) 1.5 10-12
B) 3.8 10-11
C) 1.1 109
D) 2.7 1010
E) 1.6 1013

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
16
For which of the following reactions are the numerical values of Kp and Kc the same?
1. 2SO2(g) + O2(g) 2SO3(g)
2SO3(g)
2. N2(g) + O2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2NO(g)
3. H2(g)+ I2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2HI(g)
A) 1 only
B) 2 only
C) 1 and 2
D) 2 and 3
E) 1,2,and 3
1. 2SO2(g) + O2(g)
 2SO3(g)
2SO3(g)2. N2(g) + O2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2NO(g)
3. H2(g)+ I2(g) 11ea8937_ab81_0f67_a16d_5b4ecfd630df_TB4499_11 2HI(g)
A) 1 only
B) 2 only
C) 1 and 2
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the Kc equilibrium-constant expression for the following equilibrium?

A)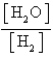
B)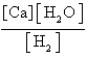
C)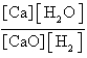
D)
E)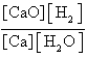

A)
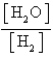
B)
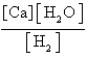
C)
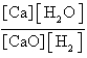
D)

E)
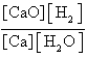

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the expression for Kc for the following equilibrium?
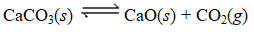
A)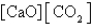
B)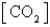
C)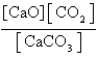
D)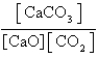
E)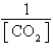
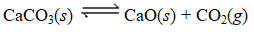
A)
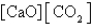
B)
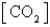
C)
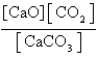
D)
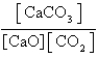
E)
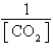

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
19
Write the expression for K for the reaction of hydrofluoric acid with water.
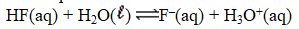
A)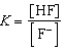
B)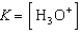
C)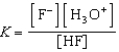
D)
E)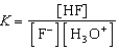
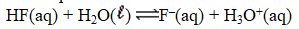
A)
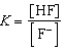
B)
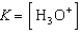
C)
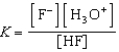
D)

E)
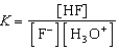

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the balanced equation for the following equilibrium expression? 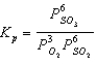
A) 6SO2(g)+ 3O2(g) 6SO3(g)
6SO3(g)
B) 6SO3(g) 6SO2(g)+ 3O2(g)
6SO2(g)+ 3O2(g)
C) 6SO3(aq) 6SO2(aq)+ 3O2(aq)
6SO2(aq)+ 3O2(aq)
D) 6SO2(aq)+ 3O2(aq) 6SO3(aq)
6SO3(aq)
E) SO2(g)+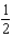 O2(g)
O2(g)  SO3(g)
SO3(g)
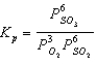
A) 6SO2(g)+ 3O2(g)
 6SO3(g)
6SO3(g)B) 6SO3(g)
 6SO2(g)+ 3O2(g)
6SO2(g)+ 3O2(g)C) 6SO3(aq)
 6SO2(aq)+ 3O2(aq)
6SO2(aq)+ 3O2(aq)D) 6SO2(aq)+ 3O2(aq)
 6SO3(aq)
6SO3(aq)E) SO2(g)+
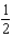 O2(g)
O2(g)  SO3(g)
SO3(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
21
If the reaction quotient,Q,is greater than K in a gas phase reaction,then
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
22
At a high temperature,equal concentrations of 0.160 mol/L of H2(g)and I2(g)are initially present in a flask.The H2 and I2 react according to the balanced equation below.
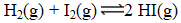
When equilibrium is reached,the concentration of H2(g)has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
A) 3.4
B) 4.0
C) 12
D) 22
E) 48
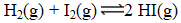
When equilibrium is reached,the concentration of H2(g)has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
A) 3.4
B) 4.0
C) 12
D) 22
E) 48

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
23
Consider the reaction
A(aq)![<strong>Consider the reaction A(aq) 2 B(aq)where K<sub>c</sub> = 4.1 at 25 <sup> \circ </sup>C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 <sup> \circ </sup>C,what change in concentrations (if any)will occur in time?</strong> A) [A] will decrease and [B] will decrease. B) [A] will decrease and [B] will increase. C) [A] will increase and [B] will decrease. D) [A] will increase and [B] will increase. E) [A] and [B] remain unchanged.](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab84_1cbd_a16d_4f59e7a5571e_TB4499_11.jpg) 2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?
2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?
A) [A] will decrease and [B] will decrease.
B) [A] will decrease and [B] will increase.
C) [A] will increase and [B] will decrease.
D) [A] will increase and [B] will increase.
E) [A] and [B] remain unchanged.
A(aq)
![<strong>Consider the reaction A(aq) 2 B(aq)where K<sub>c</sub> = 4.1 at 25 <sup> \circ </sup>C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 <sup> \circ </sup>C,what change in concentrations (if any)will occur in time?</strong> A) [A] will decrease and [B] will decrease. B) [A] will decrease and [B] will increase. C) [A] will increase and [B] will decrease. D) [A] will increase and [B] will increase. E) [A] and [B] remain unchanged.](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab84_1cbd_a16d_4f59e7a5571e_TB4499_11.jpg) 2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?
2 B(aq)where Kc = 4.1 at 25 C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 C,what change in concentrations (if any)will occur in time?A) [A] will decrease and [B] will decrease.
B) [A] will decrease and [B] will increase.
C) [A] will increase and [B] will decrease.
D) [A] will increase and [B] will increase.
E) [A] and [B] remain unchanged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
24
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g)  2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
A) 0.44
B) 2.3
C) 0.31
D) 0.78
E) 0.11
 2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?A) 0.44
B) 2.3
C) 0.31
D) 0.78
E) 0.11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following is always true for a reaction where Kc is 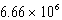 at 25 C?
at 25 C?
A) The reaction mixture contains mostly products at equilibrium.
B) The reaction mixture contains mostly reactants at equilibrium.
C) The rate of reaction is very fast.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.
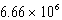 at 25 C?
at 25 C?A) The reaction mixture contains mostly products at equilibrium.
B) The reaction mixture contains mostly reactants at equilibrium.
C) The rate of reaction is very fast.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
26
For the reaction TlSCN(s)  Tl+(aq)+ SCN-(aq),Kc =
Tl+(aq)+ SCN-(aq),Kc = 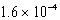
At 25 C.Which of the following concerning a 125 mL solution containing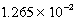
M Tl+,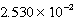
M SCN- and a large excess of TlSCN(s)is/are correct?
1)The mixture is at equilibrium.
2)Additional TlSCN(s)must precipitate to attain equilibrium.
3)The reaction quotient (Q)is greater than one.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
 Tl+(aq)+ SCN-(aq),Kc =
Tl+(aq)+ SCN-(aq),Kc = 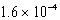
At 25 C.Which of the following concerning a 125 mL solution containing
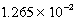
M Tl+,
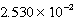
M SCN- and a large excess of TlSCN(s)is/are correct?
1)The mixture is at equilibrium.
2)Additional TlSCN(s)must precipitate to attain equilibrium.
3)The reaction quotient (Q)is greater than one.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following statements about the reaction quotient,Q,is false?
A) The value of Q can be used to predict equilibrium concentrations.
B) It has the same expression as Kc.
C) Its value is calculated using nonequilibrium concentrations.
D) If Q > Kc,the reaction must move to equilibrium by forming more reactants.
E) If Q < Kc,the reaction must move to equilibrium by forming more products.
A) The value of Q can be used to predict equilibrium concentrations.
B) It has the same expression as Kc.
C) Its value is calculated using nonequilibrium concentrations.
D) If Q > Kc,the reaction must move to equilibrium by forming more reactants.
E) If Q < Kc,the reaction must move to equilibrium by forming more products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
28
At a given temperature,0.0664 mol N2O4(g)is placed in a 1.00 L flask.After reaching equilibrium,the concentration of NO2(g)is 6.1 10-3 M.What is Kc for the reaction below?
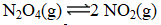
A) 3.7 10-5
B) 1.4 10-4
C) 5.9 10-4
D) 9.6 10-2
E) 1.8 103
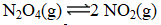
A) 3.7 10-5
B) 1.4 10-4
C) 5.9 10-4
D) 9.6 10-2
E) 1.8 103

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider the following reaction:
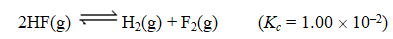
Given that 1.00 mol of HF(g),0.389 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00-L flask,determine the reaction quotient,Q.
A) Q = 0.0729
B) Q = 0.292
C) Q = 0.0584
D) Q = 2.14
E) none of these
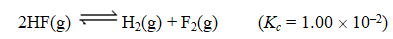
Given that 1.00 mol of HF(g),0.389 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00-L flask,determine the reaction quotient,Q.
A) Q = 0.0729
B) Q = 0.292
C) Q = 0.0584
D) Q = 2.14
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
30
At 25 C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below?
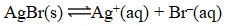
A) 5.40 10-13
B) 5.40 10-11
C) 1.90 10-8
D) 7.35 10-7
E) 1.90 10-6
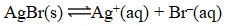
A) 5.40 10-13
B) 5.40 10-11
C) 1.90 10-8
D) 7.35 10-7
E) 1.90 10-6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
31
The reaction quotient,Q,for a system is 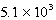 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 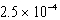
,what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net gain in both product(s)and reactant(s).
C) There will be a net gain in product(s).
D) There will be a net gain in reactant(s).
E) The equilibrium constant will decrease until it equals the reaction quotient.
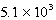 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 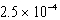
,what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net gain in both product(s)and reactant(s).
C) There will be a net gain in product(s).
D) There will be a net gain in reactant(s).
E) The equilibrium constant will decrease until it equals the reaction quotient.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
32
An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq)and 0.120 M NH3(aq).At equilibrium,the C6H5O-(aq)concentration is 0.050 M.Calculate K for the reaction.
C6H5OH(aq)+ NH3(aq)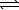 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)
A) 0.10
B) 0.24
C) 2.1
D) 4.2
E) 4.8
C6H5OH(aq)+ NH3(aq)
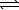 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)A) 0.10
B) 0.24
C) 2.1
D) 4.2
E) 4.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
33
Excess Ag2SO4(s)is placed in water at 25 C.At equilibrium,the solution contains 0.029 M Ag+(aq).What is the equilibrium constant for the reaction below?
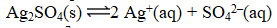
A) 1.8 10-7
B) 6.1 10-6
C) 1.2 10-5
D) 2.4 10-5
E) 8.4 10-4
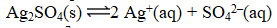
A) 1.8 10-7
B) 6.1 10-6
C) 1.2 10-5
D) 2.4 10-5
E) 8.4 10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
34
A 2.5 L flask is filled with 0.25 mol SO3,0.20 mol SO2,and 0.40 mol O2,and allowed to reach equilibrium.Assume the temperature of the mixture is chosen so that Kc = 0.12.Predict the effect on the concentration of SO3 as equilibrium is achieved by using Q,the reaction quotient.
2 SO3(g)![<strong>A 2.5 L flask is filled with 0.25 mol SO<sub>3</sub>,0.20 mol SO<sub>2</sub>,and 0.40 mol O<sub>2</sub>,and allowed to reach equilibrium.Assume the temperature of the mixture is chosen so that K<sub>c</sub> = 0.12.Predict the effect on the concentration of SO<sub>3</sub> as equilibrium is achieved by using Q,the reaction quotient. 2 SO<sub>3</sub>(g) 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)</strong> A) [SO<sub>3</sub>] will decrease because Q > K. B) [SO<sub>3</sub>] will decrease because Q < K. C) [SO<sub>3</sub>] will increase because Q < K. D) [SO<sub>3</sub>] will increase because Q > K. E) [SO<sub>3</sub>] will remain the same because Q = K.](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab83_f5ab_a16d_156fa7013855_TB4499_11.jpg) 2 SO2(g)+ O2(g)
2 SO2(g)+ O2(g)
A) [SO3] will decrease because Q > K.
B) [SO3] will decrease because Q < K.
C) [SO3] will increase because Q < K.
D) [SO3] will increase because Q > K.
E) [SO3] will remain the same because Q = K.
2 SO3(g)
![<strong>A 2.5 L flask is filled with 0.25 mol SO<sub>3</sub>,0.20 mol SO<sub>2</sub>,and 0.40 mol O<sub>2</sub>,and allowed to reach equilibrium.Assume the temperature of the mixture is chosen so that K<sub>c</sub> = 0.12.Predict the effect on the concentration of SO<sub>3</sub> as equilibrium is achieved by using Q,the reaction quotient. 2 SO<sub>3</sub>(g) 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)</strong> A) [SO<sub>3</sub>] will decrease because Q > K. B) [SO<sub>3</sub>] will decrease because Q < K. C) [SO<sub>3</sub>] will increase because Q < K. D) [SO<sub>3</sub>] will increase because Q > K. E) [SO<sub>3</sub>] will remain the same because Q = K.](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab83_f5ab_a16d_156fa7013855_TB4499_11.jpg) 2 SO2(g)+ O2(g)
2 SO2(g)+ O2(g)A) [SO3] will decrease because Q > K.
B) [SO3] will decrease because Q < K.
C) [SO3] will increase because Q < K.
D) [SO3] will increase because Q > K.
E) [SO3] will remain the same because Q = K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
35
A 10.0-g sample of solid NH4Cl is heated in a 5.00-L container to 900.°C.At equilibrium the pressure of NH3(g)is 1.47 atm.
NH4Cl(s) NH3(g)+ HCl(g)
NH3(g)+ HCl(g)
The equilibrium constant,Kp,for the reaction is:
A) 2.16
B) 7.78
C) 1.47
D) 2.94
E) none of these
NH4Cl(s)
 NH3(g)+ HCl(g)
NH3(g)+ HCl(g)The equilibrium constant,Kp,for the reaction is:
A) 2.16
B) 7.78
C) 1.47
D) 2.94
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
36
If the reaction quotient,Q,is equal to K in a gas phase reaction,then
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
37
When 0.20 mole HF is dissolved in water to a volume of 1.00 L,5.8% of the HF dissociates to form F-(aq).What is the equilibrium constant for the reaction?
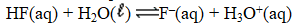
A) 1.3 10-4
B) 7.1 10-4
C) 1.2 10-2
D) 1.7 10-2
E) 6.2 10-2
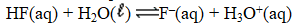
A) 1.3 10-4
B) 7.1 10-4
C) 1.2 10-2
D) 1.7 10-2
E) 6.2 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
38
Consider the following equilibrium:
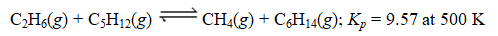
Suppose 15.6 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 45.0-L reaction vessel at 500 K.Which of the following statements is correct?
A) Because Qc < Kc,more products will be formed.
B) Because Qc = 1,the system is at equilibrium.
C) Because Qc = 1,more products will be formed.
D) Because Qc = 1,more reactants will be formed.
E) Because Qc > Kc,more reactants will be formed.
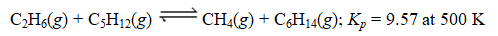
Suppose 15.6 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 45.0-L reaction vessel at 500 K.Which of the following statements is correct?
A) Because Qc < Kc,more products will be formed.
B) Because Qc = 1,the system is at equilibrium.
C) Because Qc = 1,more products will be formed.
D) Because Qc = 1,more reactants will be formed.
E) Because Qc > Kc,more reactants will be formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the reaction quotient,Q,for the equilibrium
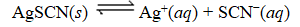 SCN-(aq)
SCN-(aq)
When 0.4257 L of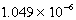
M Ag+ is combined with 0.2376 L of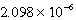
M SCN- in the presence of an excess of AgSCN(s)?
A)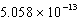
B)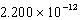
C)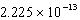
D)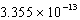
E)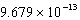
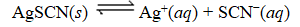 SCN-(aq)
SCN-(aq)When 0.4257 L of
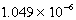
M Ag+ is combined with 0.2376 L of
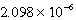
M SCN- in the presence of an excess of AgSCN(s)?
A)
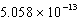
B)
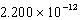
C)
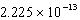
D)
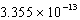
E)
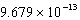

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
40
Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation:
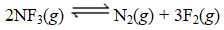
6.00-L reaction vessel is initially charged with 1.96 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0380 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L.atm.mol.K)
A) 1.53 10-5
B) 1.91 10-3
C) 1.76 10-3
D) 1.59 10-5
E) 4.43 10-7
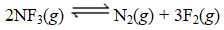
6.00-L reaction vessel is initially charged with 1.96 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0380 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L.atm.mol.K)
A) 1.53 10-5
B) 1.91 10-3
C) 1.76 10-3
D) 1.59 10-5
E) 4.43 10-7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
41
The equilibrium constant at 25 C for the dissolution of silver iodide is
8.5 10-17.AgI(s)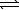 Ag+(aq)+ I-(aq)
Ag+(aq)+ I-(aq)
If an excess quantity of AgI(s)is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
A) 7.2 10-33 M
B) 4.3 10-17 M
C) 8.5 10-17 M
D) 6.5 10-9 M
E) 9.2 10-9 M
8.5 10-17.AgI(s)
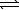 Ag+(aq)+ I-(aq)
Ag+(aq)+ I-(aq)If an excess quantity of AgI(s)is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
A) 7.2 10-33 M
B) 4.3 10-17 M
C) 8.5 10-17 M
D) 6.5 10-9 M
E) 9.2 10-9 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
42
The following reaction occurred when a 1.0-liter reaction vessel was initially charged with 2.0 moles of N2(g)and 4.0 moles of H2(g):
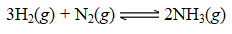
Once equilibrium was established,the concentration of NH3(g)was determined to be 0.59 M at 700.°C.The value for Kc at 700.°C for the formation of ammonia is:
A) 3.5 10-1
B) 6.8 10-3
C) 1.1 10-1
D) 6.6 10-2
E) none of these
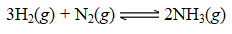
Once equilibrium was established,the concentration of NH3(g)was determined to be 0.59 M at 700.°C.The value for Kc at 700.°C for the formation of ammonia is:
A) 3.5 10-1
B) 6.8 10-3
C) 1.1 10-1
D) 6.6 10-2
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
43
Consider the following equilibrium:
CO2(g)+ H2(g) CO(g)+ H2O(g); Kc = 1.6 at 1260 K
CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.038 mol CO2 and 0.022 mol H2 are placed in a 1.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
A) 9.9 atm
B) 1.1 atm
C) 4.1 atm
D) 2.6 atm
E) 1.5 atm
CO2(g)+ H2(g)
 CO(g)+ H2O(g); Kc = 1.6 at 1260 K
CO(g)+ H2O(g); Kc = 1.6 at 1260 KSuppose 0.038 mol CO2 and 0.022 mol H2 are placed in a 1.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
A) 9.9 atm
B) 1.1 atm
C) 4.1 atm
D) 2.6 atm
E) 1.5 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
44
The equilibrium constant,Kc,for the decomposition of ammonium hydrogen sulfide is 1.8 10-4 at 25 C.NH4HS(s) 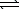 NH3(g)+ H2S(g)
NH3(g)+ H2S(g)
If excess NH4HS(s)is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
A) 3.2 10-8 M
B) 9.0 10-5 M
C) 1.8 10-4 M
D) 6.7 10-3 M
E) 1.3 10-2 M
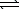 NH3(g)+ H2S(g)
NH3(g)+ H2S(g)If excess NH4HS(s)is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
A) 3.2 10-8 M
B) 9.0 10-5 M
C) 1.8 10-4 M
D) 6.7 10-3 M
E) 1.3 10-2 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
45
Nitrogen and oxygen gases may react to form nitrogen monoxide.
At 1500 C,Kc equals 1.0 10-5.N2(g)+ O2(g)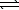 2 NO(g)
2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 C,what is the concentration of NO(g)when equilibrium is established?
A) 3.0 10-7 M
B) 4.7 10-5 M
C) 9.5 10-5 M
D) 3.0 10-2 M
E) 9.1 101 M
At 1500 C,Kc equals 1.0 10-5.N2(g)+ O2(g)
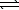 2 NO(g)
2 NO(g)If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 C,what is the concentration of NO(g)when equilibrium is established?
A) 3.0 10-7 M
B) 4.7 10-5 M
C) 9.5 10-5 M
D) 3.0 10-2 M
E) 9.1 101 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
46
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
A) 0.13 M
B) 0.37 M
C) 0.26 M
D) 1.0 M
E) 0.017 M
 PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?A) 0.13 M
B) 0.37 M
C) 0.26 M
D) 1.0 M
E) 0.017 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
47
Consider the reaction H2 + I2  2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
A) 7.6 10-2 M
B) 1.1 10-1 M
C) 3.8 10-2 M
D) 1.3 101 M
E) 5.7 10-3 M
 2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
2HI for which Kc = 43.6 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.A) 7.6 10-2 M
B) 1.1 10-1 M
C) 3.8 10-2 M
D) 1.3 101 M
E) 5.7 10-3 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
48
A 2.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.191 mol H2 and 0.191 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C?
½ H2(g)+ ½ I2(g) HI(g)
HI(g)
A) 1.23 102
B) 8.10 10-3
C) 1.72 10-2
D) 11.1
E) 7.63
½ H2(g)+ ½ I2(g)
 HI(g)
HI(g)A) 1.23 102
B) 8.10 10-3
C) 1.72 10-2
D) 11.1
E) 7.63

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
49
Nitrosyl bromide decomposes according to the chemical equation below.2 NOBr(g) 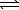 2 NO(g)+ Br2(g)
2 NO(g)+ Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
A) 2.3 10-3
B) 4.5 10-3
C) 3.5 10-2
D) 4.8 10-2
E) 8.0 10-2
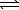 2 NO(g)+ Br2(g)
2 NO(g)+ Br2(g)When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
A) 2.3 10-3
B) 4.5 10-3
C) 3.5 10-2
D) 4.8 10-2
E) 8.0 10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
50
For the reaction given below,2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container.
A(g)+ 2B(g) C(g)
C(g)
At equilibrium,the concentration of A is 0.223 mol/L.What is the value of Kc?
A) 1.41
B) 1.77
C) 6.34
D) 0.223
E) 0.495
A(g)+ 2B(g)
 C(g)
C(g)At equilibrium,the concentration of A is 0.223 mol/L.What is the value of Kc?
A) 1.41
B) 1.77
C) 6.34
D) 0.223
E) 0.495

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
51
Sulfuryl chloride decomposes to sulfur dioxide and chlorine.
SO2Cl2(g)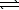 SO2(g)+ Cl2(g)
SO2(g)+ Cl2(g)
Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
A) 0.0034 M
B) 0.030 M
C) 0.040 M
D) 0.058 M
E) 0.075 M
SO2Cl2(g)
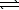 SO2(g)+ Cl2(g)
SO2(g)+ Cl2(g)Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
A) 0.0034 M
B) 0.030 M
C) 0.040 M
D) 0.058 M
E) 0.075 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
52
In an experiment,0.42 mol H2 and 0.42 mol I2 are mixed in a 1.00-L container,and the reaction forms HI.If Kc = 49.for this reaction,what is the equilibrium concentration of HI?
I2(g)+ H2(g) 2HI(g)
2HI(g)
A) 0.81 M
B) 0.74 M
C) 0.65 M
D) 0.105 M
E) 0.056 M
I2(g)+ H2(g)
 2HI(g)
2HI(g)A) 0.81 M
B) 0.74 M
C) 0.65 M
D) 0.105 M
E) 0.056 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
53
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation:
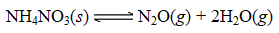
At equilibrium the total pressure in the container was found to be 2.81 atm at a temperature of 500.°C.Calculate Kp.
A) 1.75
B) 0.877
C) 3.29
D) 88.8
E) 0.822
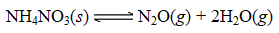
At equilibrium the total pressure in the container was found to be 2.81 atm at a temperature of 500.°C.Calculate Kp.
A) 1.75
B) 0.877
C) 3.29
D) 88.8
E) 0.822

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
54
At 25 C,the decomposition of dinitrogen tetraoxide
N2O4(g)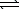 2 NO2(g)
2 NO2(g)
Has an equilibrium constant (Kp)of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
A) 0.0745 atm NO2(g)and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g)and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g)and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g)and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g)and 0.0377 N2O4(g)
N2O4(g)
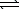 2 NO2(g)
2 NO2(g)Has an equilibrium constant (Kp)of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
A) 0.0745 atm NO2(g)and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g)and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g)and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g)and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g)and 0.0377 N2O4(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
55
A mixture of nitrogen and hydrogen was allowed to come to equilibrium at a given temperature.
3H2 + N2 2NH3
2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2,3.2 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
A) 3.2
B) 4.8
C) 5.0
D) 5.9
E) 4.4
3H2 + N2
 2NH3
2NH3An analysis of the mixture at equilibrium revealed 2.1 mol N2,3.2 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
A) 3.2
B) 4.8
C) 5.0
D) 5.9
E) 4.4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
56
For the equilibrium N2O4(g)  2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
A) 4.9 atm
B) 24 atm
C) 0.0017 atm
D) 0.0054 atm
E) 0.073 atm
 2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.6 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?A) 4.9 atm
B) 24 atm
C) 0.0017 atm
D) 0.0054 atm
E) 0.073 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
57
At 800 K,the equilibrium constant,Kp,for the following reaction is
3.2 10-7.2 H2S(g)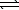 2 H2(g)+ S2(g)
2 H2(g)+ S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
A) 8.5 10-5 atm
B) 6.2 10-3 atm
C) 9.0 10-3 atm
D) 1.1 10-2 atm
E) 1.4 10-2 atm
3.2 10-7.2 H2S(g)
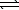 2 H2(g)+ S2(g)
2 H2(g)+ S2(g)A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
A) 8.5 10-5 atm
B) 6.2 10-3 atm
C) 9.0 10-3 atm
D) 1.1 10-2 atm
E) 1.4 10-2 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
58
At 700 K,Kp for the following equilibrium is
5.6 10-3.2HgO(s) 2Hg(l)+ O2(g)
2Hg(l)+ O2(g)
Suppose 51.2 g of mercury(II)oxide is placed in a sealed 3.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol))
A) 0.075 atm
B) 0.0056 atm
C) 4.5 atm
D) 19 atm
E) 2.3 atm
5.6 10-3.2HgO(s)
 2Hg(l)+ O2(g)
2Hg(l)+ O2(g)Suppose 51.2 g of mercury(II)oxide is placed in a sealed 3.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol))
A) 0.075 atm
B) 0.0056 atm
C) 4.5 atm
D) 19 atm
E) 2.3 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
59
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?
A) 0.46 M
B) 0.12 M
C) 0.012 M
D) 0.0026 M
E) 9.3 M
 PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.23 M,what is the equilibrium concentration of PCl5(g)?A) 0.46 M
B) 0.12 M
C) 0.012 M
D) 0.0026 M
E) 9.3 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
60
A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B.Gas A decomposes according to the following reaction:
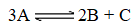
The equilibrium concentration of gas C is 0.146 mol/L.Determine the value of the equilibrium constant,Kc.
A) 0.206
B) 0.163
C) 3.84 10-3
D) 0.516
E) none of these
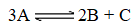
The equilibrium concentration of gas C is 0.146 mol/L.Determine the value of the equilibrium constant,Kc.
A) 0.206
B) 0.163
C) 3.84 10-3
D) 0.516
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
61
Consider the following equilibrium at 25°C:
2ICl(g) I2(g)+ Cl2(g); H = 27 kJ; Kp = 6.2 10-6
I2(g)+ Cl2(g); H = 27 kJ; Kp = 6.2 10-6
Which of the following would be true if the temperature were increased to 100°C?
1)The value of Kp would increase.
2)The concentration of ICl(g)would increase.
3)The partial pressure of I2 would increase.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3
2ICl(g)
 I2(g)+ Cl2(g); H = 27 kJ; Kp = 6.2 10-6
I2(g)+ Cl2(g); H = 27 kJ; Kp = 6.2 10-6Which of the following would be true if the temperature were increased to 100°C?
1)The value of Kp would increase.
2)The concentration of ICl(g)would increase.
3)The partial pressure of I2 would increase.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
62
At a given temperature,K = 0.021 for the equilibrium:
PCl5(g) PCl3(g)+ Cl2(g)
PCl3(g)+ Cl2(g)
What is K for: Cl2(g)+ PCl3(g) PCl5(g)?
PCl5(g)?
A) 2300
B) 21
C) 0.00044
D) 48
E) 0.021
PCl5(g)
 PCl3(g)+ Cl2(g)
PCl3(g)+ Cl2(g)What is K for: Cl2(g)+ PCl3(g)
 PCl5(g)?
PCl5(g)?A) 2300
B) 21
C) 0.00044
D) 48
E) 0.021

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
63
Given the equilibrium constants for the equilibria,
2NH4+(aq)+ 2H2O(l) 2NH3(aq)+ 2H3O+(aq); Kc =
2NH3(aq)+ 2H3O+(aq); Kc = 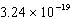
CH3COOH(aq)+ H2O(l) CH3COO-(aq)+ H3O+(aq); Kc =
CH3COO-(aq)+ H3O+(aq); Kc = 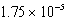
Determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq) CH3COO-(aq)+ NH4+(aq)
CH3COO-(aq)+ NH4+(aq)
A) 3.08 104
B) 3.25 10-5
C) 9.96 10-15
D) 1.00 1014
E) 1.75 10-5
2NH4+(aq)+ 2H2O(l)
 2NH3(aq)+ 2H3O+(aq); Kc =
2NH3(aq)+ 2H3O+(aq); Kc = 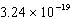
CH3COOH(aq)+ H2O(l)
 CH3COO-(aq)+ H3O+(aq); Kc =
CH3COO-(aq)+ H3O+(aq); Kc = 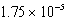
Determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq)
 CH3COO-(aq)+ NH4+(aq)
CH3COO-(aq)+ NH4+(aq)A) 3.08 104
B) 3.25 10-5
C) 9.96 10-15
D) 1.00 1014
E) 1.75 10-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
64
Assume that the following chemical reaction is at equilibrium.
C(s)+ H2O(g)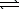 H2(g)+ CO(g)
H2(g)+ CO(g)
Which of the following statements is/are CORRECT?
1)Increasing the concentration of H2(g)will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g).
2)Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
3)Increasing the amount of C(s)will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
C(s)+ H2O(g)
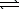 H2(g)+ CO(g)
H2(g)+ CO(g)Which of the following statements is/are CORRECT?
1)Increasing the concentration of H2(g)will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g).
2)Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
3)Increasing the amount of C(s)will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g).
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
65
For the reaction N2O4(g)  2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction?
2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction?
14NO2(g) 7N2O4(g)
7N2O4(g)
A) 6.43 105
B) 1.04
C) 1.56 10-6
D) 0.965
E) 6.76
 2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction?
2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction?14NO2(g)
 7N2O4(g)
7N2O4(g)A) 6.43 105
B) 1.04
C) 1.56 10-6
D) 0.965
E) 6.76

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
66
If Kc = 0.152 for A2 + 2B  2AB,what is the value of Kc for the reaction
2AB,what is the value of Kc for the reaction
4AB 2A2 + 4B?
2A2 + 4B?
A) 0.152
B) 0.304
C) 43.3
D) -0.152
E) 3.29
 2AB,what is the value of Kc for the reaction
2AB,what is the value of Kc for the reaction4AB
 2A2 + 4B?
2A2 + 4B?A) 0.152
B) 0.304
C) 43.3
D) -0.152
E) 3.29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
67
The symbol Q is called the ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
68
The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows.
N2(g)+ 3 H2(g)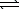 2 NH3(g) H = -92.2 kJ
2 NH3(g) H = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)
N2(g)+ 3 H2(g)
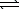 2 NH3(g) H = -92.2 kJ
2 NH3(g) H = -92.2 kJGiven a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
69
Given the following chemical equilibria,
N2(g)+ O2(g)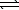 2 NO(g) K1
2 NO(g) K1
N2(g)+ 3 H2(g)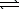 2 NH3(g) K2
2 NH3(g) K2
H2(g)+ 1/2 O2(g)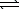 H2O(g) K3
H2O(g) K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g)+ 5 O2(g)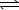 4 NO(g)+ 6 H2O(g) K c
4 NO(g)+ 6 H2O(g) K c
A)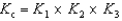
B)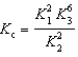
C)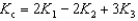
D)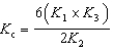
E)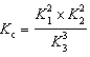
N2(g)+ O2(g)
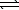 2 NO(g) K1
2 NO(g) K1N2(g)+ 3 H2(g)
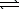 2 NH3(g) K2
2 NH3(g) K2H2(g)+ 1/2 O2(g)
 H2O(g) K3
H2O(g) K3Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g)+ 5 O2(g)
 4 NO(g)+ 6 H2O(g) K c
4 NO(g)+ 6 H2O(g) K cA)
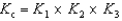
B)
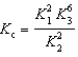
C)
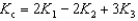
D)
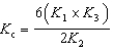
E)
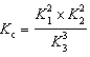

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
70
A flask contains the following chemical system at equilibrium.
CuCO3(s) Cu2+(aq)+ 2 CO32-(aq)
Cu2+(aq)+ 2 CO32-(aq)
Addition of which of the following substances will increase the solubility of CuCO3(s)in water?
1)aqueous hydrochloric acid
2)aqueous sodium carbonate
3)solid copper(II)carbonate
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
CuCO3(s)
 Cu2+(aq)+ 2 CO32-(aq)
Cu2+(aq)+ 2 CO32-(aq)Addition of which of the following substances will increase the solubility of CuCO3(s)in water?
1)aqueous hydrochloric acid
2)aqueous sodium carbonate
3)solid copper(II)carbonate
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following equilibria would not be affected by pressure changes at constant temperature?
A) CO2(g)+ H2(g) CO(g)+ H2O(g)
CO(g)+ H2O(g)
B) CO(g)+ O2(g)
O2(g)  CO2(g)
CO2(g)
C) 2Hg(l)+ O2(g) 2HgO(s)
2HgO(s)
D) 2H2(g)+ O2(g) 2H2O(l)
2H2O(l)
E) CaCO3(s) CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
A) CO2(g)+ H2(g)
 CO(g)+ H2O(g)
CO(g)+ H2O(g)B) CO(g)+
 O2(g)
O2(g)  CO2(g)
CO2(g)C) 2Hg(l)+ O2(g)
 2HgO(s)
2HgO(s)D) 2H2(g)+ O2(g)
 2H2O(l)
2H2O(l)E) CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
72
Consider the following equilibrium:
PCl5(g) PCl3(g)+ Cl2(g); H = 92 kJ
PCl3(g)+ Cl2(g); H = 92 kJ
The concentration of PCl3 at equilibrium may be increased by
A) decreasing the temperature.
B) adding Cl2 to the system.
C) adding PCl5 to the system.
D) increasing the pressure.
E) adding a catalyst.
PCl5(g)
 PCl3(g)+ Cl2(g); H = 92 kJ
PCl3(g)+ Cl2(g); H = 92 kJThe concentration of PCl3 at equilibrium may be increased by
A) decreasing the temperature.
B) adding Cl2 to the system.
C) adding PCl5 to the system.
D) increasing the pressure.
E) adding a catalyst.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
73
Given the following equilibria,
PbBr2(s) Pb2+(aq)+ 2 Br-(aq) K1 = 6.6 0 10-6
Pb2+(aq)+ 2 Br-(aq) K1 = 6.6 0 10-6
Pb(OH)2(s) Pb2+(aq)+ 2 OH-(aq) K2 = 1.4 10-15
Pb2+(aq)+ 2 OH-(aq) K2 = 1.4 10-15
Determine the equilibrium constant,Kc,for the following reaction.
PbBr2(s)+ 2 OH-(aq) Pb(OH)2(s)+ 2 Br-(aq)
Pb(OH)2(s)+ 2 Br-(aq)
A) 9.2 10-21
B) 2.1 10-10
C) 6.6 10-6
D) 4.7 109
E) 1.1 1020
PbBr2(s)
 Pb2+(aq)+ 2 Br-(aq) K1 = 6.6 0 10-6
Pb2+(aq)+ 2 Br-(aq) K1 = 6.6 0 10-6Pb(OH)2(s)
 Pb2+(aq)+ 2 OH-(aq) K2 = 1.4 10-15
Pb2+(aq)+ 2 OH-(aq) K2 = 1.4 10-15Determine the equilibrium constant,Kc,for the following reaction.
PbBr2(s)+ 2 OH-(aq)
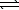 Pb(OH)2(s)+ 2 Br-(aq)
Pb(OH)2(s)+ 2 Br-(aq)A) 9.2 10-21
B) 2.1 10-10
C) 6.6 10-6
D) 4.7 109
E) 1.1 1020

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
74
Given the following equilibria,
Ni2+(aq)+ 2 OH-(aq)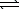 Ni(OH)2(s) K1 = 1.8 1015
Ni(OH)2(s) K1 = 1.8 1015
Ni2+(aq)+ 4 CN-(aq)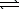 Ni(CN)42-(aq) K2 = 2.0 1031
Ni(CN)42-(aq) K2 = 2.0 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq)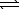 Ni(CN)42-(aq)+ 2 OH-(aq)
Ni(CN)42-(aq)+ 2 OH-(aq)
A) 2.8 10-47
B) 9.0 10-17
C) 1.8 1015
D) 1.1 1016
E) 3.6 1046
Ni2+(aq)+ 2 OH-(aq)
 Ni(OH)2(s) K1 = 1.8 1015
Ni(OH)2(s) K1 = 1.8 1015Ni2+(aq)+ 4 CN-(aq)
 Ni(CN)42-(aq) K2 = 2.0 1031
Ni(CN)42-(aq) K2 = 2.0 1031Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq)
 Ni(CN)42-(aq)+ 2 OH-(aq)
Ni(CN)42-(aq)+ 2 OH-(aq)A) 2.8 10-47
B) 9.0 10-17
C) 1.8 1015
D) 1.1 1016
E) 3.6 1046

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
75
In which of the following reactions does a decrease in the volume of the reaction vessel at constant temperature favor formation of the products?
A) 2H2(g)+ O2(g) 2H2O(g)
2H2O(g)
B) NO2(g)+ CO(g) NO(g)+ CO2(g)
NO(g)+ CO2(g)
C) H2(g)+ I2(g) 2HI(g)
2HI(g)
D) 2O3(g) 3O2(g)
3O2(g)
E) MgCO3(s) MgO(s)+ CO2(g)
MgO(s)+ CO2(g)
A) 2H2(g)+ O2(g)
 2H2O(g)
2H2O(g)B) NO2(g)+ CO(g)
 NO(g)+ CO2(g)
NO(g)+ CO2(g)C) H2(g)+ I2(g)
 2HI(g)
2HI(g)D) 2O3(g)
 3O2(g)
3O2(g)E) MgCO3(s)
 MgO(s)+ CO2(g)
MgO(s)+ CO2(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
76
Assume that the following chemical reaction is at equilibrium.
2 ICl(g) I2(g)+ Cl2(g) H = +26.9 kJ
I2(g)+ Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.
2 ICl(g)
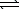 I2(g)+ Cl2(g) H = +26.9 kJ
I2(g)+ Cl2(g) H = +26.9 kJAt 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
77
When the pressure of an equilibrium mixture of SO2,O2,and SO3 is doubled at constant temperature,what the effect on Kp?
2SO2(g)+ O2(g) 2SO3(g)
2SO3(g)
A) Kp is halved.
B) Kp is doubled.
C) Kp is unchanged.
D) Kp is tripled.
E) Kp is decreased by a third.
2SO2(g)+ O2(g)
 2SO3(g)
2SO3(g)A) Kp is halved.
B) Kp is doubled.
C) Kp is unchanged.
D) Kp is tripled.
E) Kp is decreased by a third.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
78
In 1913,the Haber-Bosch process was patented.The product of the Haber-Bosch process is ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
79
Given the equilibrium constants for the following reactions:
4Cu(s)+ O2(g) 2Cu2O(s),K1
2Cu2O(s),K1
4CuO(s) 2Cu2O(s)+ O2(g),K2
2Cu2O(s)+ O2(g),K2
What is K for the system
2Cu(s)+ O2(g) 2CuO(s)
2CuO(s)
Equivalent to?
A)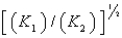
B)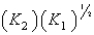
C) (K1)(K2)
D)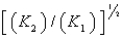
E)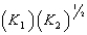
4Cu(s)+ O2(g)
 2Cu2O(s),K1
2Cu2O(s),K14CuO(s)
 2Cu2O(s)+ O2(g),K2
2Cu2O(s)+ O2(g),K2What is K for the system
2Cu(s)+ O2(g)
 2CuO(s)
2CuO(s)Equivalent to?
A)
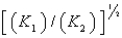
B)
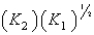
C) (K1)(K2)
D)
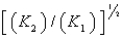
E)
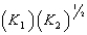

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck
80
If a stress is applied to an equilibrium system,the system will respond in such a way as to relieve that stress.This is a statement of ________ principle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 82 في هذه المجموعة.
فتح الحزمة
k this deck








