Deck 7: The Structure of Atoms
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/73
العب
ملء الشاشة (f)
Deck 7: The Structure of Atoms
1
A light emitting diode (L.E.D.)emits photons with an energy of 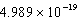 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted?
A)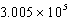 J/mol
J/mol
B)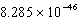 J/mol
J/mol
C)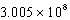 J/mol
J/mol
D)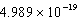 J/mol
J/mol
E)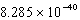 J/mol
J/mol
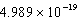 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted?A)
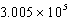 J/mol
J/molB)
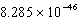 J/mol
J/molC)
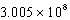 J/mol
J/molD)
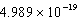 J/mol
J/molE)
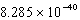 J/mol
J/mol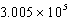 J/mol
J/mol 2
A device operates at a frequency of 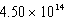 Hz.What is the wavelength of this radiation?
Hz.What is the wavelength of this radiation?
A) 667 nm
B)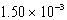 nm
nm
C)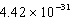 nm
nm
D) 0.0895 nm
E)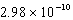 nm
nm
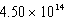 Hz.What is the wavelength of this radiation?
Hz.What is the wavelength of this radiation?A) 667 nm
B)
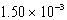 nm
nmC)
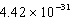 nm
nmD) 0.0895 nm
E)
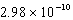 nm
nm667 nm
3
What is the wavelength of a photon having a frequency of 11.7 THz? (1 THz = 1015 Hz)
A) 0.0390 nm
B) 7.76 10-24 nm
C) 2.33 10-15 nm
D) 25.6 nm
E) 2.56 1016 nm
A) 0.0390 nm
B) 7.76 10-24 nm
C) 2.33 10-15 nm
D) 25.6 nm
E) 2.56 1016 nm
25.6 nm
4
According to experiments concerned with the photoelectric effect,which of the following will increase the kinetic energy of an electron ejected from a metal surface?
1)increasing the wavelength of the light striking the surface
2)increasing the frequency of the light striking the surface
3)increasing the number of photons of light striking the surface
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)increasing the wavelength of the light striking the surface
2)increasing the frequency of the light striking the surface
3)increasing the number of photons of light striking the surface
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the energy of a photon of electromagnetic radiation with a wavelength of 451.7 nm?
A)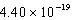 J
J
B)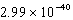 J
J
C)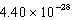 J
J
D)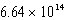 J
J
E)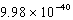 J
J
A)
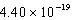 J
JB)
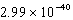 J
JC)
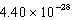 J
JD)
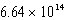 J
JE)
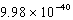 J
J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements is/are CORRECT?
1)FM radio waves have longer wavelengths than visible radiation and AM radio waves have shorter wavelengths than visible radiation.
2)Magnetic resonance imaging (MRI)uses a mixture of x-rays and gamma rays.
3)Exposure to ultraviolet (UV)radiation can lead to sunburns.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)FM radio waves have longer wavelengths than visible radiation and AM radio waves have shorter wavelengths than visible radiation.
2)Magnetic resonance imaging (MRI)uses a mixture of x-rays and gamma rays.
3)Exposure to ultraviolet (UV)radiation can lead to sunburns.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following ranks regions of the electromagnetic spectrum in proper order from highest to lowest frequency.
A) radio > ultraviolet> x-rays > visible > microwaves
B) x-rays > ultraviolet > visible > microwaves> radio
C) microwaves > x-rays > ultraviolet > visible > radio
D) ultraviolet > x-rays > microwaves > visible > radio
E) visible > microwaves > radio > ultraviolet > x-rays
A) radio > ultraviolet> x-rays > visible > microwaves
B) x-rays > ultraviolet > visible > microwaves> radio
C) microwaves > x-rays > ultraviolet > visible > radio
D) ultraviolet > x-rays > microwaves > visible > radio
E) visible > microwaves > radio > ultraviolet > x-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the energy per mole of photons of light with a wavelength of 976.9 nm?
A)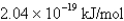
B)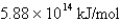
C)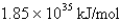
D)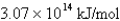
E)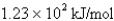
A)
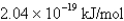
B)
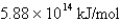
C)
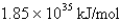
D)
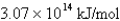
E)
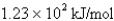

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following regions of the electromagnetic spectrum has the lowest frequency?
A) x-ray
B) gamma ray
C) ultraviolet
D) infrared
E) visible
A) x-ray
B) gamma ray
C) ultraviolet
D) infrared
E) visible

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
10
A red laser pointer emits light at a wavelength of 488 nm.If the laser emits 7.5 10-4 J of energy per second in the form of visible radiation,how many photons per second are emitted from the laser?
A) 4.1 10-19 photons/sec
B) 5.4 10-16 photons/sec
C) 8.9 1014 photons/sec
D) 1.8 1015 photons/sec
E) 2.5 1018 photons/sec
A) 4.1 10-19 photons/sec
B) 5.4 10-16 photons/sec
C) 8.9 1014 photons/sec
D) 1.8 1015 photons/sec
E) 2.5 1018 photons/sec

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the frequency of gamma ray radiation that has a wavelength of 11.4 pm?
A) 3.80 10-20 s-1
B) 1.74 10-14 s-1
C) 2.63 107 s-1
D) 3.42 109 s-1
E) 2.63 1019 s-1
A) 3.80 10-20 s-1
B) 1.74 10-14 s-1
C) 2.63 107 s-1
D) 3.42 109 s-1
E) 2.63 1019 s-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
12
A device emits light at 761.7 nm.What is the frequency of this radiation?
A) 3.94 10-4 Hz
B) 1.15 1027 Hz
C) 3.94 1014 Hz
D) 2.61 10-37 Hz
E) 2.61 10-19 Hz
A) 3.94 10-4 Hz
B) 1.15 1027 Hz
C) 3.94 1014 Hz
D) 2.61 10-37 Hz
E) 2.61 10-19 Hz

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
13
The ____ of a photon of light is ____ proportional to its frequency and ____ proportional to its wavelength.
A) energy,directly,inversely
B) energy,inversely,directly
C) velocity,directly,inversely
D) intensity,inversely,directly
E) amplitude,directly,inversely
A) energy,directly,inversely
B) energy,inversely,directly
C) velocity,directly,inversely
D) intensity,inversely,directly
E) amplitude,directly,inversely

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the wavelength of a photon that has an energy of 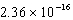 J?
J?
A)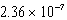 nm
nm
B)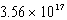 nm
nm
C) 0.842 nm
D)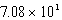 nm
nm
E)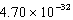 nm
nm
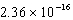 J?
J?A)
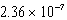 nm
nmB)
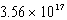 nm
nmC) 0.842 nm
D)
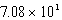 nm
nmE)
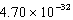 nm
nm
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
15
If the energy of 1.00 mole of photons is 245 kJ,what is the wavelength of the light?
A) 122 nm
B) 488 nm
C) 1220 nm
D) 787 nm
E) 811 nm
A) 122 nm
B) 488 nm
C) 1220 nm
D) 787 nm
E) 811 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the energy of a photon of electromagnetic radiation with a frequency of 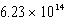 Hz?
Hz?
A)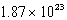 J
J
B)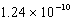 J
J
C)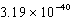 J
J
D)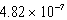 J
J
E)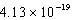 J
J
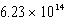 Hz?
Hz?A)
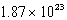 J
JB)
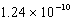 J
JC)
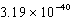 J
JD)
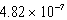 J
JE)
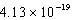 J
J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the energy per mole of photons of light with a frequency of 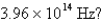
A)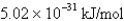
B)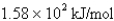
C)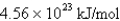
D)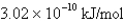
E)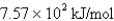
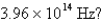
A)
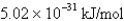
B)
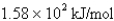
C)
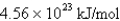
D)
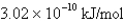
E)
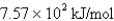

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the binding energy of an electron in a photosensitive metal (in kJ/mol)if the longest wavelength of light that can eject electrons from the metal is 205 nm?
A) 279 kJ/mol
B) 584 kJ/mol
C) 792 kJ/mol
D) 1.71 1019 kJ/mol
E) 5.84 10-20 kJ/mol
A) 279 kJ/mol
B) 584 kJ/mol
C) 792 kJ/mol
D) 1.71 1019 kJ/mol
E) 5.84 10-20 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
19
If a cordless phone operates at a frequency of 9.00 108 s-1.What is the wavelength of this radiation?
A) 0.333 m
B) 1.99 10-25 m
C) 3.33 10-9 m
D) 3.71 10-18 m
E) 2.70 1017 m
A) 0.333 m
B) 1.99 10-25 m
C) 3.33 10-9 m
D) 3.71 10-18 m
E) 2.70 1017 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following colors of visible light has the highest frequency?
A) green
B) orange
C) red
D) yellow
E) blue
A) green
B) orange
C) red
D) yellow
E) blue

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which type of experiment demonstrates that an electron has the properties of a wave?
A) nuclear fission
B) electron diffraction
C) light emission from atomic gases
D) mass spectroscopy
E) photoelectric effect
A) nuclear fission
B) electron diffraction
C) light emission from atomic gases
D) mass spectroscopy
E) photoelectric effect

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
22
For which of the following transitions would a hydrogen atom emit the lowest energy photon?
A) n = 4 to n = 5
B) n = 5 to n = 2
C) n = 4 to n = 3
D) n = 3 to n = 1
E) n = 3 to n = 2
A) n = 4 to n = 5
B) n = 5 to n = 2
C) n = 4 to n = 3
D) n = 3 to n = 1
E) n = 3 to n = 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
23
If the de Broglie wavelength of an electron is 44 nm,what is its velocity? The mass of an electron is 9.11 10-31 kg.
A) 6.0 10-5 m/s
B) 1.2 103 m/s
C) 1.7 104 m/s
D) 3.1 1010 m/s
E) 4.8 1022 m/s
A) 6.0 10-5 m/s
B) 1.2 103 m/s
C) 1.7 104 m/s
D) 3.1 1010 m/s
E) 4.8 1022 m/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
24
For which of the following electron transitions would a hydrogen atom emit a photon of the highest energy?
A)
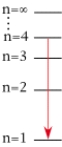
B)
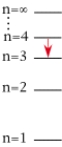
C)
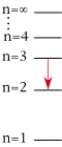
D)
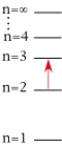
A)
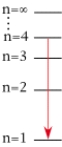
B)
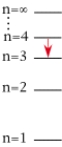
C)
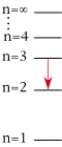
D)
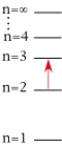

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
25
For which of the following transitions would a hydrogen atom absorb a photon with the longest wavelength?
A) n = 1 to n = 2
B) n = 4 to n = 6
C) n = 5 to n = 4
D) n = 7 to n = 6
E) n = 6 to n = 7
A) n = 1 to n = 2
B) n = 4 to n = 6
C) n = 5 to n = 4
D) n = 7 to n = 6
E) n = 6 to n = 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which type of experiment demonstrates that light has the properties of a particle?
A) nuclear fission
B) electron diffraction
C) light emission from atomic gases
D) mass spectroscopy
E) photoelectric effect
A) nuclear fission
B) electron diffraction
C) light emission from atomic gases
D) mass spectroscopy
E) photoelectric effect

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
27
According to the Bohr model for the hydrogen atom,the energy necessary to excite an electron from n = 7 to n = 8 is ____ the energy necessary to excite an electron from n = 5 to n = 6.
A) less than
B) greater than
C) equal to
D) either equal to or greater than
E) either less than or equal to
A) less than
B) greater than
C) equal to
D) either equal to or greater than
E) either less than or equal to

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
28
The electron in a hydrogen atom,originally in level 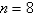 ,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? (
,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? ( 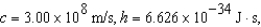
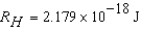 )
)
A) 5
B) 6
C) 8
D) 9
E) 1
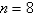 ,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? (
,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? ( 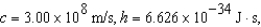
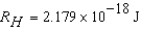 )
)A) 5
B) 6
C) 8
D) 9
E) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
29
The energy required to break one mole of hydrogen-hydrogen bonds in H2 is 436 kJ/mol.What is the longest wavelength of light capable of breaking a single H-H bond?
A) 0.474 nm
B) 119 nm
C) 132 nm
D) 274 nm
E) 474 nm
A) 0.474 nm
B) 119 nm
C) 132 nm
D) 274 nm
E) 474 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the de Broglie wavelength of a 148-g baseball traveling at 97.1 mph? ( 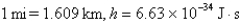 )
)
A)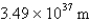
B)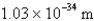
C)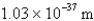
D)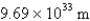
E)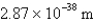
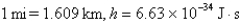 )
)A)
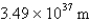
B)
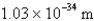
C)
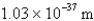
D)
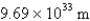
E)
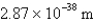

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following is/are correct postulates of Bohr's theory of the hydrogen atom ?
1)The energy of an electron in an atom is quantized (i.e.only specific energy values are possible).
2)The principal quantum number (n),specifies each unique energy level.
3)An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The energy of an electron in an atom is quantized (i.e.only specific energy values are possible).
2)The principal quantum number (n),specifies each unique energy level.
3)An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following statements is INCORRECT?
A) It is not possible to know the exact location of an electron and its exact energy simultaneously.
B) The energies of an atom's electrons are quantized.
C) Quantum numbers define the energy states and the orbitals available to an electron.
D) The behavior of an atom's electrons can be described by circular orbits around a nucleus.
E) Electrons have both wave and particle properties.
A) It is not possible to know the exact location of an electron and its exact energy simultaneously.
B) The energies of an atom's electrons are quantized.
C) Quantum numbers define the energy states and the orbitals available to an electron.
D) The behavior of an atom's electrons can be described by circular orbits around a nucleus.
E) Electrons have both wave and particle properties.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
33
The contribution for which de Broglie is best remembered in modern science is
A) his statement that no electron can have identical values for all four quantum numbers.
B) his proposal that particles of matter should be associated with wavelike behavior.
C) his statement that an electron can exist in an atom only in discrete energy levels.
D) his statement that elements show periodic repetition of properties.
E) his statement that electrons occupy all the orbitals of a given sublevel singly before pairing begins.
A) his statement that no electron can have identical values for all four quantum numbers.
B) his proposal that particles of matter should be associated with wavelike behavior.
C) his statement that an electron can exist in an atom only in discrete energy levels.
D) his statement that elements show periodic repetition of properties.
E) his statement that electrons occupy all the orbitals of a given sublevel singly before pairing begins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
34
Erwin Schrödinger developed a model for the behavior of electrons in atoms that is known as quantum mechanics.Which of the following statements concerning this model is/are CORRECT?
1)The energy of an electron is quantized.
2)The energy of an electron is equal to its mass multiplied by the square of its velocity.
3)Electrons travel in circular orbits around a nucleus.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The energy of an electron is quantized.
2)The energy of an electron is equal to its mass multiplied by the square of its velocity.
3)Electrons travel in circular orbits around a nucleus.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes a transition from level
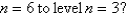 (
( 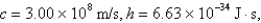
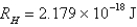 )
)
A)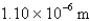
B)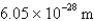
C)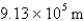
D)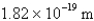
E)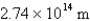
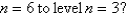 (
( 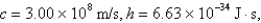
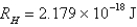 )
)A)
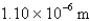
B)
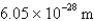
C)
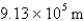
D)
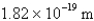
E)
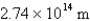

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
36
If a hydrogen atom in the excited n = 4 state relaxes to the ground state,what is the maximum number of possible emission lines?
A) 1
B) 3
C) 6
D) 8
E) infinite
A) 1
B) 3
C) 6
D) 8
E) infinite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
37
For a proton (mass = 1.673 10-27 kg)moving with a velocity of 2.83 104 m/s,what is the de Broglie wavelength (in pm)?
A) 0.356 pm
B) 3.56 pm
C) 14.0 pm
D) 7.15 pm
E) 28.5 pm
A) 0.356 pm
B) 3.56 pm
C) 14.0 pm
D) 7.15 pm
E) 28.5 pm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
38
In Bohr's atomic theory,when an electron moves from one energy level to another energy level more distant from the nucleus,
A) energy is absorbed.
B) light is emitted.
C) energy is emitted.
D) no change in energy occurs.
E) none of these
A) energy is absorbed.
B) light is emitted.
C) energy is emitted.
D) no change in energy occurs.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following statements concerning quantum mechanics is/are true?
1)The behavior of submicroscopic particles can sometimes be described as waves.
2)Quantum mechanics limits us to making statistical statements about the location of an electron in an atom.
3)The uncertainty principle is important only for particles of very small mass,such as the electron.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)The behavior of submicroscopic particles can sometimes be described as waves.
2)Quantum mechanics limits us to making statistical statements about the location of an electron in an atom.
3)The uncertainty principle is important only for particles of very small mass,such as the electron.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the de Broglie wavelength of an electron traveling at 9.47% of the speed of light? ( 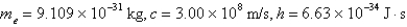 )
)
A)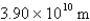
B)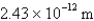
C)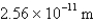
D)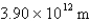
E)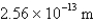
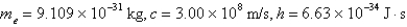 )
)A)
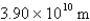
B)
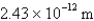
C)
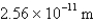
D)
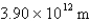
E)
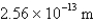

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
41
All of the following sets of quantum numbers are allowed EXCEPT
A) n = 6,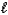 = 5,
= 5, 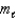 = 1
= 1
B) n = 6,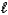 = 3,
= 3, 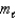 = 3
= 3
C) n = 2,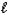 = 0,
= 0, 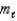 = 0
= 0
D) n = 5,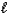 = 4,
= 4, 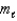 = 6
= 6
E) n = 5,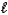 = 4,
= 4, 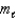 = 3
= 3
A) n = 6,
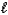 = 5,
= 5, 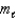 = 1
= 1B) n = 6,
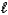 = 3,
= 3, 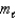 = 3
= 3C) n = 2,
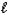 = 0,
= 0, 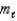 = 0
= 0D) n = 5,
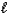 = 4,
= 4, 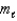 = 6
= 6E) n = 5,
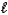 = 4,
= 4, 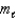 = 3
= 3
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
42
A 4p orbital has ?
A) 3 planar nodes and 1 spherical node.
B) 0 planar nodes and 2 spherical nodes.
C) 1 planar node and 2 spherical nodes.
D) 1 planar node and 0 spherical nodes.
E) 2 planar nodes and 3 spherical nodes.
A) 3 planar nodes and 1 spherical node.
B) 0 planar nodes and 2 spherical nodes.
C) 1 planar node and 2 spherical nodes.
D) 1 planar node and 0 spherical nodes.
E) 2 planar nodes and 3 spherical nodes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the value of the orbital angular momentum quantum number ( 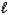 )for an electron in a 5s orbital?
)for an electron in a 5s orbital?
A) 2
B) 4
C) 3
D) 0
E) 1
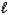 )for an electron in a 5s orbital?
)for an electron in a 5s orbital?A) 2
B) 4
C) 3
D) 0
E) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following sets of quantum numbers refers to a 4d orbital?
A) n = 2,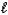 = 1,
= 1, 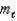 = -1
= -1
B) n = 2,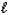 = 4,
= 4, 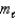 = -1
= -1
C) n = 4,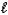 = 2,
= 2, 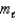 = -1
= -1
D) n = 4,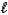 = 3,
= 3, 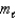 = 0
= 0
E) n = 4,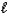 = 3,
= 3, 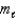 = +2
= +2
A) n = 2,
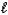 = 1,
= 1, 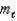 = -1
= -1B) n = 2,
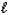 = 4,
= 4, 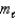 = -1
= -1C) n = 4,
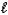 = 2,
= 2, 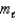 = -1
= -1D) n = 4,
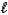 = 3,
= 3, 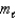 = 0
= 0E) n = 4,
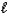 = 3,
= 3, 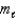 = +2
= +2
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
45
The n = ____ shell is the lowest that may contain s-orbitals.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the following properties is associated with the value of the 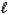 quantum number?
quantum number?
A) the shape of an orbital
B) the size of an orbital
C) the number of electrons in an orbital
D) the energy of an orbital
E) the orientation in space of an orbital
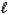 quantum number?
quantum number?A) the shape of an orbital
B) the size of an orbital
C) the number of electrons in an orbital
D) the energy of an orbital
E) the orientation in space of an orbital

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which type of orbital is designated n = 1 and 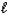 = 0?
= 0?
A) 1s
B) 2s
C) 3p
D) 4d
E) 1p
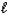 = 0?
= 0?A) 1s
B) 2s
C) 3p
D) 4d
E) 1p

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following properties is associated with the value of the n quantum number?
A) the number of electrons in an orbital
B) the size of an orbital
C) the orientation in space of an orbital
D) the energy of an orbital
E) the shape of an orbital
A) the number of electrons in an orbital
B) the size of an orbital
C) the orientation in space of an orbital
D) the energy of an orbital
E) the shape of an orbital

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many values are there for the magnetic quantum number ( 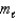 )when the value of the angular momentum quantum number (
)when the value of the angular momentum quantum number ( 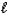 )is 4?
)is 4?
A) 14
B) 9
C) 2
D) 4
E) 15
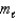 )when the value of the angular momentum quantum number (
)when the value of the angular momentum quantum number ( 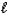 )is 4?
)is 4?A) 14
B) 9
C) 2
D) 4
E) 15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
50
What type of orbital is designated n = 4, 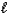 = 3,
= 3, 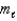 = -2?
= -2?
A) 4s
B) 4p
C) 4d
D) 4f
E) none
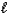 = 3,
= 3, 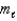 = -2?
= -2?A) 4s
B) 4p
C) 4d
D) 4f
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following orbital boundary surfaces represent d-orbitals? 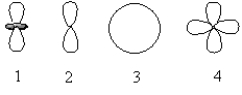
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 4

A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the total number of orbitals having n = 4 and 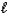 = 0?
= 0?
A) 1
B) 3
C) 5
D) 7
E) 10
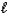 = 0?
= 0?A) 1
B) 3
C) 5
D) 7
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
53
How many p orbitals are in the n = 3 shell?
A) 1
B) 6
C) 0
D) 5
E) 3
A) 1
B) 6
C) 0
D) 5
E) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
54
A possible value of the magnetic quantum number 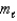 for a 5d electron is
for a 5d electron is
A) 2.
B) -4.
C) 5.
D) -6.
E) 3.
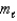 for a 5d electron is
for a 5d electron isA) 2.
B) -4.
C) 5.
D) -6.
E) 3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the total number of orbitals found in the n = 4 shell?
A) 16
B) 4
C) 20
D) 24
E) 15
A) 16
B) 4
C) 20
D) 24
E) 15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following orbital boundary surfaces is a representation of a 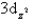 orbital?
orbital?
A)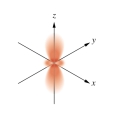
B)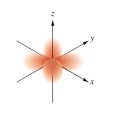
C)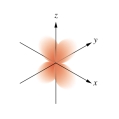
D)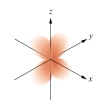
E)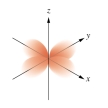
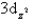 orbital?
orbital?A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following statements is/are CORRECT?
1)The angular momentum quantum number,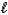
,of an electron in an s orbital is always equal to 0.
2)The magnetic quantum number,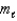
,of an electron in a p orbital is +1,0,or -1.
3)The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The angular momentum quantum number,
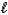
,of an electron in an s orbital is always equal to 0.
2)The magnetic quantum number,
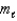
,of an electron in a p orbital is +1,0,or -1.
3)The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
58
How many orbitals have the following set of quantum numbers: n = 5, 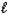 = 1,
= 1, 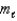 = -1?
= -1?
A) 0
B) 1
C) 3
D) 6
E) 7
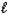 = 1,
= 1, 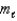 = -1?
= -1?A) 0
B) 1
C) 3
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
59
What type of orbital is designated n = 4, 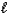 = 2,
= 2, 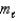 = 0?
= 0?
A) 4f
B) 4d
C) 4p
D) 4g
E) 4s
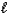 = 2,
= 2, 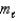 = 0?
= 0?A) 4f
B) 4d
C) 4p
D) 4g
E) 4s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following is a representation of a 3dxz orbital?
A)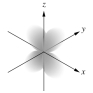
B)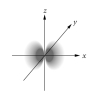
C)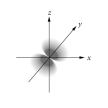
D)
E)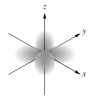
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
61
A ________,designated by the Greek symbol ,describes the wave behavior of an electron in an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
62
According to Heisenberg's ________ principle,it is impossible to simultaneously measure the exact location and energy of an electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
63
The difference in energy between adjacent energy levels in an atom _____ as n increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
64
What evidence does the photoelectric effect provide that photons are not only waves?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following statements is/are CORRECT?
1)A paramagnetic substance is attracted to a magnetic field.
2)An atom with no unpaired electrons is ferromagnetic.
3)Atoms with one or more unpaired electrons are paramagnetic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)A paramagnetic substance is attracted to a magnetic field.
2)An atom with no unpaired electrons is ferromagnetic.
3)Atoms with one or more unpaired electrons are paramagnetic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following sets of quantum numbers (n,l,ml, ms)is not permissible?
A) 3 3 -3 +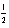
B) 2 1 -1 +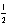
C) 2 0 0 +
D) 2 1 0 +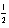
E) 4 2 -1 -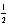
A) 3 3 -3 +
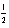
B) 2 1 -1 +
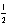
C) 2 0 0 +

D) 2 1 0 +
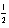
E) 4 2 -1 -
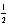

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
67
The Bohr model predicts that the energy of an atom's electron is ________,meaning that the electron can only occupy orbitals of specific energies.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the value of the spin quantum number for an electron in a 4p orbital?
A) 4
B) 1
C) either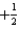
Or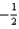
D)
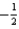
E)
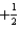
A) 4
B) 1
C) either
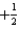
Or
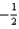
D)
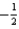
E)
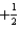

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
69
Occupied states or energy levels in the hydrogen atom with n > 1 are called _____ states.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
70
The ________ quantum number is given the symbol 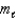
.This quantum number is related to the orientation in space of an orbital.
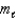
.This quantum number is related to the orientation in space of an orbital.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
71
A point in a standing wave that has zero amplitude is called a(n)________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
72
The size of an electron orbital is often chosen to be the distance within which 90% of the electron density is found.Why is the orbital radius not chosen to be the distance within which 100% of the electron density is found?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following statements is/are CORRECT?
1)A diamagnetic substance is strongly attracted to a magnetic field.
2)Substances that retain their magnetism after they are withdrawn from a magnetic field are called ferromagnetic.
3)Most transition metals and all lanthanide metals are ferromagnetic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)A diamagnetic substance is strongly attracted to a magnetic field.
2)Substances that retain their magnetism after they are withdrawn from a magnetic field are called ferromagnetic.
3)Most transition metals and all lanthanide metals are ferromagnetic.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck








