Deck 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/185
العب
ملء الشاشة (f)
Deck 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene
1
Due to electron delocalization,one would predict that the carbon-oxygen bond in acetamide,CH3CONH2
A)is nonpolar.
B)has more double bond character than the carbon-oxygen bond of acetone,(CH3)2CO.
C)is longer than the carbon-oxygen bond of dimethyl ether,(CH3)2O.
D)is longer than the carbon-oxygen bond of acetone,(CH3)2CO.
E)is formed by overlapping sp3 orbitals.
A)is nonpolar.
B)has more double bond character than the carbon-oxygen bond of acetone,(CH3)2CO.
C)is longer than the carbon-oxygen bond of dimethyl ether,(CH3)2O.
D)is longer than the carbon-oxygen bond of acetone,(CH3)2CO.
E)is formed by overlapping sp3 orbitals.
is longer than the carbon-oxygen bond of acetone,(CH3)2CO.
2
Mark the most electron-rich carbon atom in the compound below with an asterisk.

3
Which of the following pairs are resonance structures? 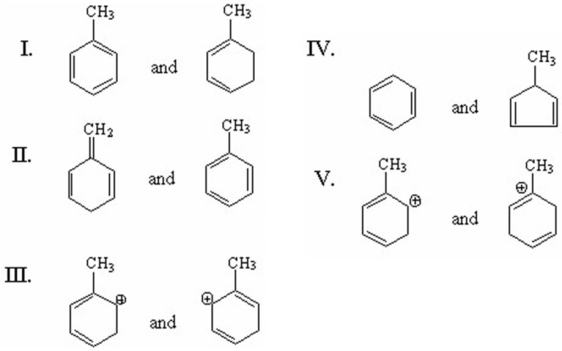
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
III
4
Does the following structures represent different compounds or resonance contributors? 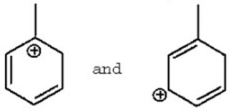


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements about benzene is correct?
A)All of the carbon atoms are sp3 hybridized.
B)It has no delocalized electrons.
C)The carbon-carbon bond length is longer than that of ethane.
D)It is a planar molecule.
E)The carbon-hydrogen bonds are not the same length.
A)All of the carbon atoms are sp3 hybridized.
B)It has no delocalized electrons.
C)The carbon-carbon bond length is longer than that of ethane.
D)It is a planar molecule.
E)The carbon-hydrogen bonds are not the same length.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
6
Draw the important resonance contributing forms for the structure shown below. 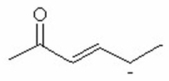


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
7
In which of the following does resonance delocalization of electron density not play a role?
A)allyl cation
B)benzyl anion
C)CO32-
D)O3
E)cyclohexyl radical
A)allyl cation
B)benzyl anion
C)CO32-
D)O3
E)cyclohexyl radical

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
8
Draw the important resonance structure of 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
9
Identify the resonance hybrid.
A)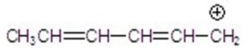
B)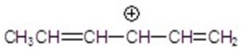
C)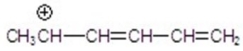
D)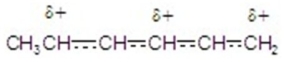
E)A,B,and C.
A)
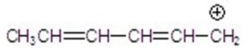
B)
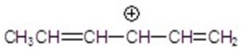
C)
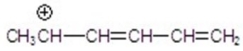
D)
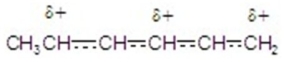
E)A,B,and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
10
Draw the important resonance contributing forms for the structure shown below. 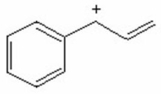


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
11
Give the hybridization,shape,and bond angle of a carbon in benzene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
12
Draw two other resonance contributors for the following compound. 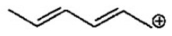


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following statements is incorrect about benzene?
A)All of the carbon atoms are sp hybridized.
B)It has delocalized electrons.
C)The carbon-carbon bond lengths are all the same.
D)The carbon-hydrogen bond lengths are all the same.
E)All twelve atoms lie in the same plane.
A)All of the carbon atoms are sp hybridized.
B)It has delocalized electrons.
C)The carbon-carbon bond lengths are all the same.
D)The carbon-hydrogen bond lengths are all the same.
E)All twelve atoms lie in the same plane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is not a resonance structure of the species shown? 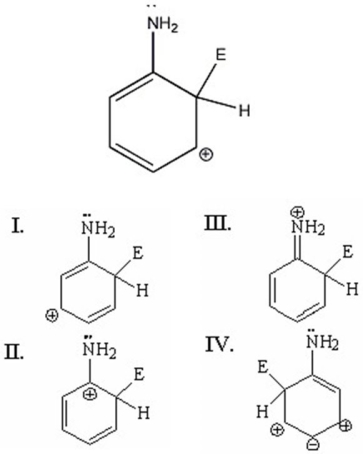
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following pairs are resonance structures? 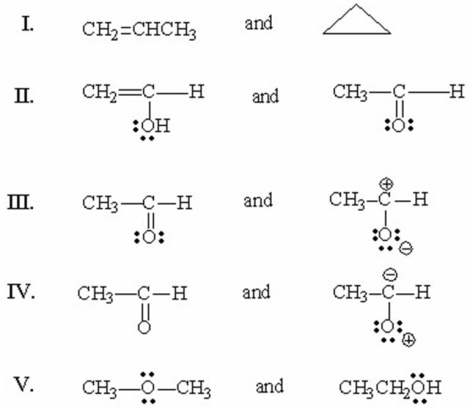
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
16
The delocalized π system in benzene is formed by a cyclic overlap of 6 ________ orbitals.
A)s
B)p
C)sp
D)sp2
E)sp3
A)s
B)p
C)sp
D)sp2
E)sp3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
17
Draw the important resonance structures of 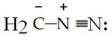
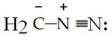

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
18
Draw the arrows to go from one resonance structure to another. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
19
Draw the important resonance structures of 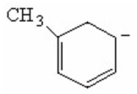


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
20
Draw the important resonance contributing forms for the structure shown below. 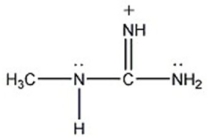


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following is the strongest acid? 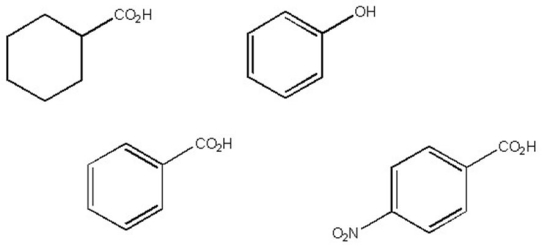


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
22
Identify the most stable structure(s).
A)CH2 CH-OCH3
CH-OCH3
B)CH2-CH OCH3
OCH3
C)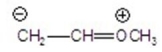
D)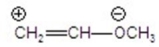
E)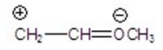
A)CH2
 CH-OCH3
CH-OCH3B)CH2-CH
 OCH3
OCH3C)

D)
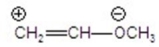
E)
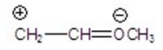

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following is the most stable resonance contributor to acetic acid? 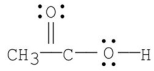
A)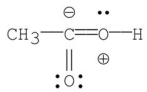
B)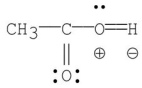
C)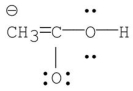
D)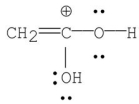
E)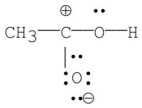
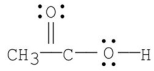
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following statements concerning resonance contributors and resonance hybrids is not correct?
A)The fewer nearly equivalent resonance contributors are in the structure,the greater the resonance energy.
B)A resonance hybrid is more stable than the predicted stability of any of its resonance contributors.
C)The greater the number of relatively stable resonance contributors,the greater the resonance energy.
D)The greater the predicted stability of a resonance contributor,the more it contributes to the resonance hybrid.
E)none of the above
A)The fewer nearly equivalent resonance contributors are in the structure,the greater the resonance energy.
B)A resonance hybrid is more stable than the predicted stability of any of its resonance contributors.
C)The greater the number of relatively stable resonance contributors,the greater the resonance energy.
D)The greater the predicted stability of a resonance contributor,the more it contributes to the resonance hybrid.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
25
Draw all major resonance contributors of the species below. 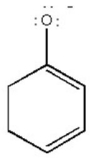


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
26
What is suggested by the fact that benzene's molar heat of hydrogenation is 36 kcal less than three times the molar heat of hydrogenation of cyclohexene?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
27
Provide the major organic product of the following reaction. 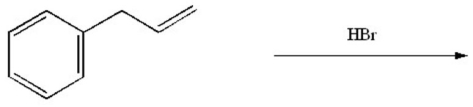


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
28
Identify the correctly drawn arrows.
A)
B)
C)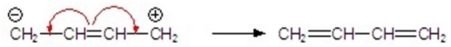
D)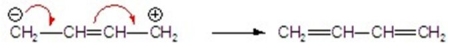
E)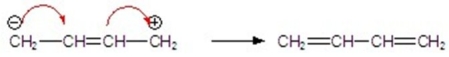
A)

B)

C)
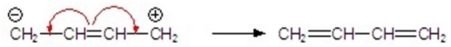
D)
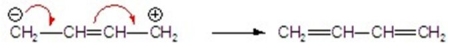
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
29
Aromatic molecules contain ________ π electrons.
A)no
B)4n + 2 (with n an integer)
C)4n + 2 (where n = 0.5)
D)4n (with n an integer)
E)unpaired
A)no
B)4n + 2 (with n an integer)
C)4n + 2 (where n = 0.5)
D)4n (with n an integer)
E)unpaired

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
30
In the acetate ion (CH3CO2-),which of the oxygen atoms bears a greater negative charge? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
31
Draw all major resonance contributors of the species below. 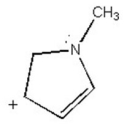


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
32
Draw the arrows to go from one resonance structure to another. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
33
Stabilization of a charged species usually happens when this species can be more accurately depicted as a hybrid of several resonance contributing forms.Why is this the case?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
34
Provide the major organic product of the following reaction. 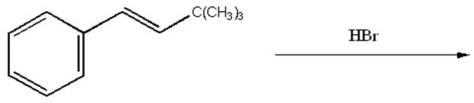


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
35
Rate the following molecules in decreasing order of stability. 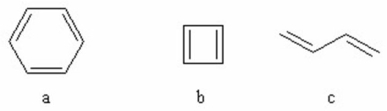


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
36
The following molecule is not aromatic.Why? 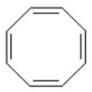


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
37
Draw all major resonance contributors of the species below. 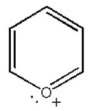


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
38
Is the following molecule aromatic? Explain. 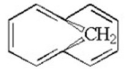


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
39
Draw four resonance contributors for the following structure and indicate which is the most important contributor.Explain. 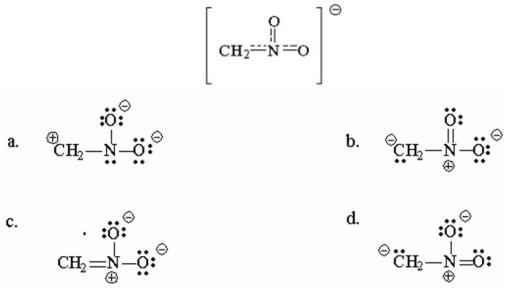


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
40
Draw all major resonance contributors of the species below. 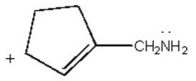


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
41
Classify the compound below as aromatic,antiaromatic,or nonaromatic.Assume planarity of the π network. 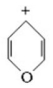


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
42
Classify cyclopheptatrienyl cation as aromatic,antiaromatic,or nonaromatic.Assume planarity of the π network.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
43
Classify the compound below as aromatic,antiaromatic,or nonaromatic.Assume planarity of the π network. 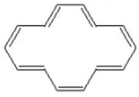


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following compounds is aromatic? 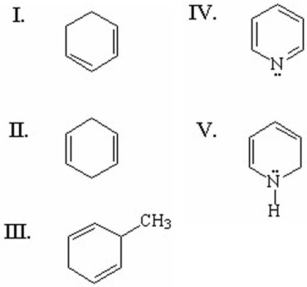
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following is aromatic? 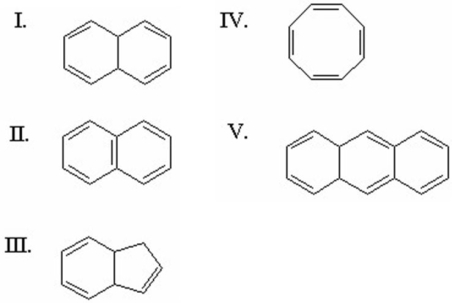
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
46
Provide the structure of the biologically significant heterocycle pyrimidine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
47
Is cyclooctatetraene a planar molecule? Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following is an aromatic hydrocarbon? 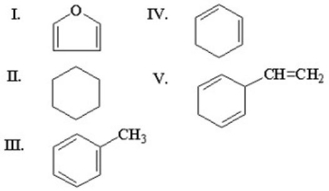
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
49
Classify cyclopentadienyl cation as aromatic,antiaromatic,or nonaromatic.Assume planarity of the π network.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
50
Provide the structure of indole.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following is aromatic? 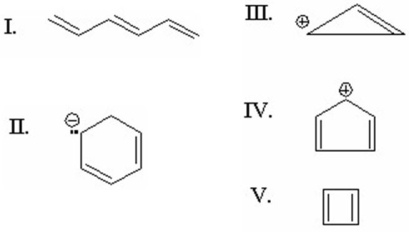
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
52
When cycloheptatriene is deprotonated,an anion with seven resonance forms of equal energy can be drawn.Given this fact,explain why cycloheptatriene is only slightly more acidic than propene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the structures below would be aromatic? 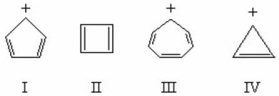
A)I and IV
B)I,III,and IV
C)III and IV
D)II

A)I and IV
B)I,III,and IV
C)III and IV
D)II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
54
Provide the structure of the biologically significant heterocycle purine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
55
Is the following molecule aromatic? Explain. 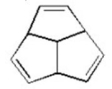


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
56
Cyclic hydrocarbons which can be represented as structures containing alternating single and double bonds are called ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following is aromatic? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
58
One of the following compounds is aromatic and the other is antiaromatic.Which is which? 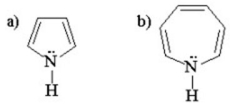


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following is aromatic?
A)cyclopentadienyl cation
B)1,3-cyclohexadiene
C)cyclobutenyl anion
D)1,3,5-hexatriene
E)cycloheptatrienyl cation
A)cyclopentadienyl cation
B)1,3-cyclohexadiene
C)cyclobutenyl anion
D)1,3,5-hexatriene
E)cycloheptatrienyl cation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following is antiaromatic? 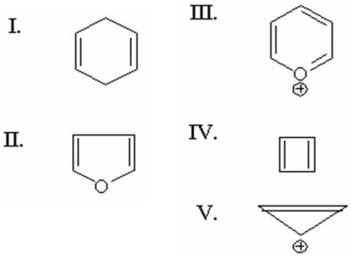
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
61
What descriptive term is applied to the type of diene represented by 2,4-hexadiene?
A)conjugated diene
B)cumulated diene
C)isolated diene
D)alkynyl diene
E)none of the above
A)conjugated diene
B)cumulated diene
C)isolated diene
D)alkynyl diene
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following compounds contains the longest carbon-carbon single bond?
A)allene
B)1,3-butadiyne
C)1,3-butadiene
D)propyne
E)ethane
A)allene
B)1,3-butadiyne
C)1,3-butadiene
D)propyne
E)ethane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following is a benzylic cation? 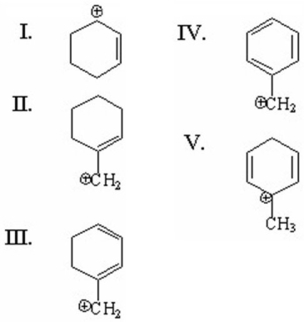
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
64
Among a series of isomeric trienes,the more negative the ΔH° of the hydrogenation reaction of a given triene,the ________ stable it is relative to the others in the isomeric series.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following is an allylic cation? 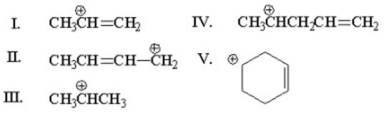
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
66
Draw the distribution of π electrons in the molecular orbitals of cyclobutadiene. 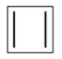


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which of the following is the most stable cation? 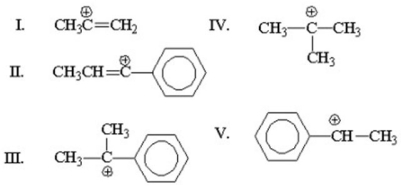
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the hybridization of the central carbon of allene (1,2-propadiene)?
A)sp
B)sp2
C)sp3
D)p
E)none of the above
A)sp
B)sp2
C)sp3
D)p
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
69
Rank the following dienes in order of increasing stability: trans-1,3-pentadiene,cis-1,3-pentadiene,1,4-pentadiene,and 1,2-pentadiene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following numbered hydrogens is most easily abstracted by heterolytic cleavage? Explain. 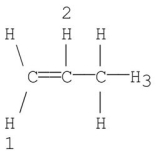


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
71
Indicate whether the following molecule is aromatic,antiaromatic,or nonaromatic. 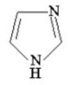


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the following compounds is more stable? Explain. 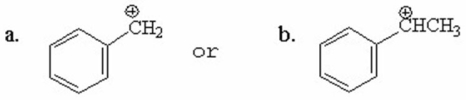
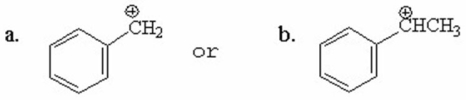

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following is the most stable diene? 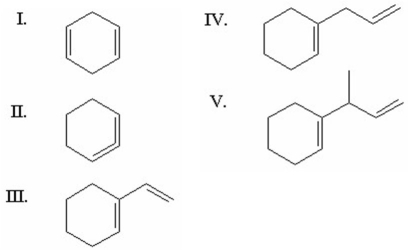
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following is a cumulated diene? 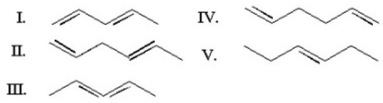
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
75
How many allylic hydrogen atoms are present in the following molecule? 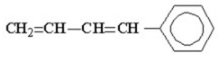
A)2
B)3
C)4
D)5
E)zero
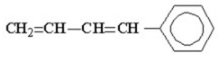
A)2
B)3
C)4
D)5
E)zero

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which species is represented by the following distribution of p electrons in the molecular energy diagram? 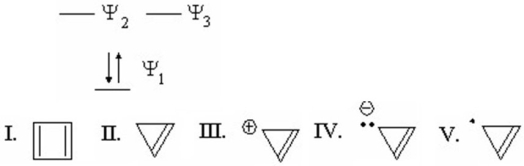
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which of the following is a conjugated diene? 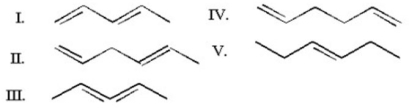
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following statements about the π molecular orbital description of cyclobutadiene is not correct?
A)Cyclobutadiene has two degenerate nonbonding π molecular orbitals.
B)Cyclobutadiene has a single bonding π molecular orbital.
C)Cyclobutadiene has two electrons in nonbonding π molecular orbitals.
D)Cyclobutadiene has one electron in an antibonding π molecular orbital which makes it antiaromatic.
E)none of the above
A)Cyclobutadiene has two degenerate nonbonding π molecular orbitals.
B)Cyclobutadiene has a single bonding π molecular orbital.
C)Cyclobutadiene has two electrons in nonbonding π molecular orbitals.
D)Cyclobutadiene has one electron in an antibonding π molecular orbital which makes it antiaromatic.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
79
What descriptive term is applied to the type of diene represented by 1,5-octadiene?
A)conjugated diene
B)cumulated diene
C)isolated diene
D)alkynyl diene
E)none of the above
A)conjugated diene
B)cumulated diene
C)isolated diene
D)alkynyl diene
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck
80
How many benzylic hydrogens are present in the following molecule? 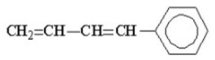
A)2
B)3
C)4
D)5
E)zero
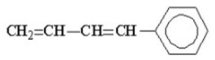
A)2
B)3
C)4
D)5
E)zero

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 185 في هذه المجموعة.
فتح الحزمة
k this deck








