Deck 9: Substitution and Elimination Reactions of Alkyl Halides
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/228
العب
ملء الشاشة (f)
Deck 9: Substitution and Elimination Reactions of Alkyl Halides
1
Assuming no other changes,what is the effect of doubling both the alkyl halide and the nucleophile concentrations in the above reaction?
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
quadruples the rate
2
Which of the following best describes the carbon-chlorine bond of an alkyl chloride?
A)nonpolar; no dipole
B)polar; δ+ at carbon and δ- at chlorine
C)polar; δ- at carbon and δ+ at chlorine
D)ionic
E)none of the above
A)nonpolar; no dipole
B)polar; δ+ at carbon and δ- at chlorine
C)polar; δ- at carbon and δ+ at chlorine
D)ionic
E)none of the above
polar; δ+ at carbon and δ- at chlorine
3
Identify the strongest nucleophile in an SN2 reaction.
A)isopropoxide ion
B)tert-butoxide ion
C)ethoxide ion
D)tert-pentoxide ion
A)isopropoxide ion
B)tert-butoxide ion
C)ethoxide ion
D)tert-pentoxide ion
ethoxide ion
4
Which of the following is not normally considered to be a nucleophile?
A)NH3
B)NH2CH3
C)HC C:-
C:-
D)CH3CH2+
A)NH3
B)NH2CH3
C)HC
 C:-
C:-D)CH3CH2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the nucleophile in the reaction shown below? 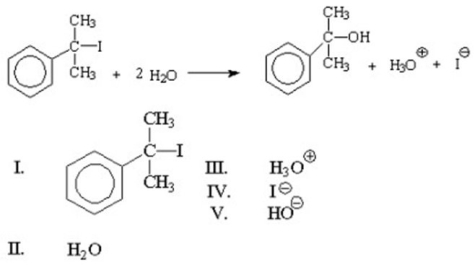
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following alkyl halides gives the slowest SN2 reaction?
A)CH3CH2Cl
B)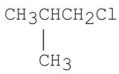
C)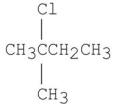
D)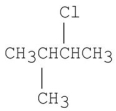
E)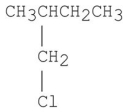
A)CH3CH2Cl
B)
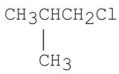
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
7
Give the mechanism including the transition state. 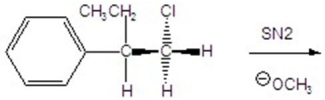


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
8
Assuming no other changes,what is the effect of doubling only the concentration of the alkyl halide in the above SN2 reaction?
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
9
Identify the alkyl halide that reacts the fastest in a SN2 reaction.
A)2-chloro-2-methylpropane
B)2-chlorobutane
C)1-chlorobutane
D)chloromethane
A)2-chloro-2-methylpropane
B)2-chlorobutane
C)1-chlorobutane
D)chloromethane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following SN2 reactions is the fastest?
A)CH3CH2CH2CH2Br + OH- → CH3CH2CH2CH2OH + Br-
B)CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + HBr
C)CH3CH2CHBrCH3 + OH- → CH3CH2CHOHCH3 + Br-
D)CH3CH2CHBrCH3 + H2O → CH3CH2CHOHCH3 + HBr
A)CH3CH2CH2CH2Br + OH- → CH3CH2CH2CH2OH + Br-
B)CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + HBr
C)CH3CH2CHBrCH3 + OH- → CH3CH2CHOHCH3 + Br-
D)CH3CH2CHBrCH3 + H2O → CH3CH2CHOHCH3 + HBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following SN2 reactions is the slowest?
A)CH3CH2CH3Br + HO- → CH3CH2CH3OH + Br-
B)CH3CH2CH2Cl + HO- → CH3CH2CH2OH + Cl-
C)CH3CH2CH2I + HO- → CH3CH2CH2OH + I-
D)CH3CH2CH3F + HO- → CH3CH2CH2OH + F-
E)
A)CH3CH2CH3Br + HO- → CH3CH2CH3OH + Br-
B)CH3CH2CH2Cl + HO- → CH3CH2CH2OH + Cl-
C)CH3CH2CH2I + HO- → CH3CH2CH2OH + I-
D)CH3CH2CH3F + HO- → CH3CH2CH2OH + F-
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
12
Identify the alkyl halide that reacts the fastest in an SN2 reaction.
A)1-chloropropane
B)1-bromopropane
C)1-fluoropropane
D)1-iodopropane
A)1-chloropropane
B)1-bromopropane
C)1-fluoropropane
D)1-iodopropane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following SN2 reactions is the fastest?
A)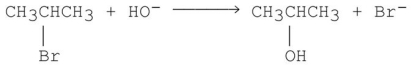
B)CH3CH2CH2I + HO- → CH3CH2CH2OH + I-
C)
D)CH3CH2CH3Br + HO- → CH3CH2CH3OH + Br-
E)CH3CH2CH2I + H2O → CH3CH2CH2OH + HI
A)

B)CH3CH2CH2I + HO- → CH3CH2CH2OH + I-
C)

D)CH3CH2CH3Br + HO- → CH3CH2CH3OH + Br-
E)CH3CH2CH2I + H2O → CH3CH2CH2OH + HI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is not a nucleophile?
A)CH3NH2
B)PH3
C)(+CH3)
D)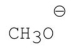
E)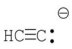
A)CH3NH2
B)PH3
C)(+CH3)
D)
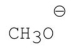
E)
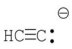

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following compounds will undergo an SN2 reaction most readily?
A)(CH3)3CCH2I
B)(CH3)3CCl
C)(CH3)2CHI
D)(CH3)2CHCH2CH2CH2Cl
E)(CH3)2CHCH2CH2CH2I
A)(CH3)3CCH2I
B)(CH3)3CCl
C)(CH3)2CHI
D)(CH3)2CHCH2CH2CH2Cl
E)(CH3)2CHCH2CH2CH2I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is a secondary alkyl halide?
A)CH3Br
B)(CH3)3CBr
C)(CH3)2CHBr
D)(CH3)2CHCH2Br
A)CH3Br
B)(CH3)3CBr
C)(CH3)2CHBr
D)(CH3)2CHCH2Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following does not provide evidence that there are two different mechanisms for nucleophilic substitution?
A)reaction products when CH3I is used as the substrate
B)reaction products when (CH3)3CCH2I is used as substrate
C)the stereochemistry of nucleophilic substitutions
D)the effect of nucleophile concentration on rate
A)reaction products when CH3I is used as the substrate
B)reaction products when (CH3)3CCH2I is used as substrate
C)the stereochemistry of nucleophilic substitutions
D)the effect of nucleophile concentration on rate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
18
Give the mechanism including the transition state. 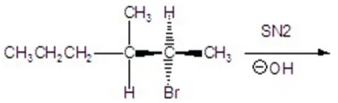


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following SN2 reactions is the slowest?
A)CH3CH2CHBrCH3 + OH- → CH3CH2CHOHCH3 + Br-
B)CH3CH2CHBrCH3 + H2O → CH3CH2CHOHCH3 + HBr
C)CH3CH2CH2CH2Br + OH- → CH3CH2CH2CH2OH + Br-
D)CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + HBr
A)CH3CH2CHBrCH3 + OH- → CH3CH2CHOHCH3 + Br-
B)CH3CH2CHBrCH3 + H2O → CH3CH2CHOHCH3 + HBr
C)CH3CH2CH2CH2Br + OH- → CH3CH2CH2CH2OH + Br-
D)CH3CH2CH2CH2Br + H2O → CH3CH2CH2CH2OH + HBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following are the substitution products of the reaction shown below? CH3CH2Br + -OH → ?
A)CH3CH2BrH+ + O-
B)HOCH2CH2Br
C)CH3CH2OH + Br-
D)CH2 CH2 + Br- + H2O
CH2 + Br- + H2O
E)CH2 CHBr + H2O
CHBr + H2O
A)CH3CH2BrH+ + O-
B)HOCH2CH2Br
C)CH3CH2OH + Br-
D)CH2
 CH2 + Br- + H2O
CH2 + Br- + H2OE)CH2
 CHBr + H2O
CHBr + H2O
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
21
Provide the structure of the major organic product of the following reaction. 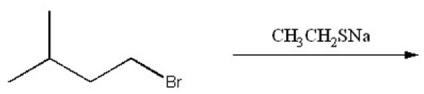


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following species is the least nucleophilic?
A)(CH3)3CO-
B)H2O
C)(CH3)3N
D)BF3
E)CN-
A)(CH3)3CO-
B)H2O
C)(CH3)3N
D)BF3
E)CN-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following species is most reactive in an SN2 reaction?
A)CH3CH2-Cl
B)CH3CH2-Br
C)CH3CH2-I
D)CH3CH2-F
E)CH3CH2-OH
A)CH3CH2-Cl
B)CH3CH2-Br
C)CH3CH2-I
D)CH3CH2-F
E)CH3CH2-OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
24
Provide the major organic product(s)in the reaction below. 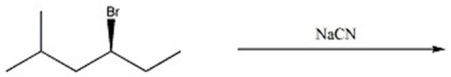
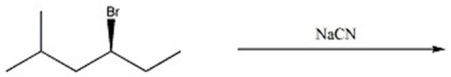

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following is the strongest nucleophile in an aqueous solution?
A)HO-
B)F-
C)Cl-
D)Br-
E)I-
A)HO-
B)F-
C)Cl-
D)Br-
E)I-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
26
Provide the structure of the major organic product of the following reaction. 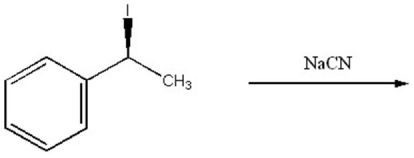


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following statements concerning SN2 reactions of alkyl halides is not correct?
A)The rate of reaction depends on the concentration of the nucleophile.
B)The rate of reaction depends on the concentration of the alkyl halide.
C)The rate of reaction of a particular alkyl bromide depends on the steric accessibility of the carbon of the C-Br bond.
D)All alkyl iodides react more rapidly than all alkyl chlorides.
E)The rate of reaction depends on the relative nucleophilicity of the nucleophile.
A)The rate of reaction depends on the concentration of the nucleophile.
B)The rate of reaction depends on the concentration of the alkyl halide.
C)The rate of reaction of a particular alkyl bromide depends on the steric accessibility of the carbon of the C-Br bond.
D)All alkyl iodides react more rapidly than all alkyl chlorides.
E)The rate of reaction depends on the relative nucleophilicity of the nucleophile.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
28
Draw and describe the transition state in the SN2 reaction between CH3I and CH3CH2O-Na+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following is the best leaving group?
A)HO-
B)F-
C)Cl-
D)Br-
E)I-
A)HO-
B)F-
C)Cl-
D)Br-
E)I-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
30
In an SN2 reaction why does the nucleophile attack the carbon on the side opposite the leaving group?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
31
Provide the structure of the major organic product of the following reaction. 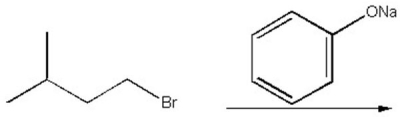


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
32
What product results from the SN2 reaction between (R)-2-chloropentane and hydroxide?
A)(R)-2-pentanol
B)(S)-2-pentanol
C)racemic pentanol
D)1-pentanol
E)3-pentanol
A)(R)-2-pentanol
B)(S)-2-pentanol
C)racemic pentanol
D)1-pentanol
E)3-pentanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
33
Provide the structure of the major organic product in the following reaction.
(CH3)3N + CH3CH2CH2I →
(CH3)3N + CH3CH2CH2I →

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
34
Provide the structure of the major organic product which results when (S)-2-iodopentane is treated with KCN in DMF.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
35
Provide the major organic product(s)in the reaction below. 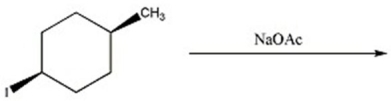


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
36
Provide a detailed,stepwise mechanism for the reaction below.
(CH3)2CHCH2CH2CH2I + CN- → (CH3)2CHCH2CH2CH2CN + I-
(CH3)2CHCH2CH2CH2I + CN- → (CH3)2CHCH2CH2CH2CN + I-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
37
Do all primary alkyl iodides undergo SN2 reactions with sodium cyanide in DMSO at identical rates? Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
38
Provide the structure of the major organic product in the following reaction. 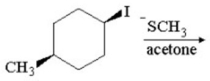


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
39
Provide the major organic product(s)in the reaction below. 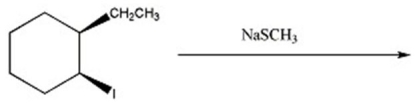


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following is the best nucleophile in water?
A)I-
B)CH3SCH3
C)CH3OCH3
D)Cl-
A)I-
B)CH3SCH3
C)CH3OCH3
D)Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
41
A student attempted to prepare 1-chlorobutane by treating 1-butanol with NaCl in acetone.Was the student successful? Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
42
Provide the major organic product(s)of the reaction shown. 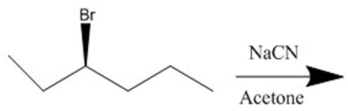


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the following bromides reacts readily via an SN2 reaction with NaN3?
A)C6H5Br
B)CH3CH2CH=CHBr
C)(C6H5)3CBr
D)(CH3)3CCH2CH2CH2Br
E)1-bromo-1-methylcyclohexane
A)C6H5Br
B)CH3CH2CH=CHBr
C)(C6H5)3CBr
D)(CH3)3CCH2CH2CH2Br
E)1-bromo-1-methylcyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
44
In each of the pairs below,which is the best nucleophile in alcoholic solvents?
a.CH3S- or CH3O-
b.(CH3)2NH or (CH3)3N
c.Cl- or F-
d.SCN- or OCN-
a.CH3S- or CH3O-
b.(CH3)2NH or (CH3)3N
c.Cl- or F-
d.SCN- or OCN-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
45
Give the mechanism. 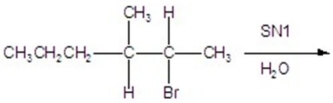


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
46
Provide the major organic product(s)of the reaction shown. 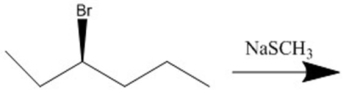


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which halide reacts most rapidly via an SN2 mechanism?
A)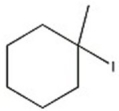
B)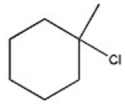
C)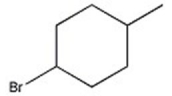
D)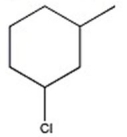
E)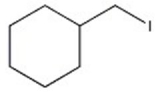
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
48
Identify the alkyl halide that reacts the fastest in an SN1 reaction.
A)2-chloropropane
B)2-bromopropane
C)2-fluoropropane
D)2-iodopropane
A)2-chloropropane
B)2-bromopropane
C)2-fluoropropane
D)2-iodopropane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
49
Assuming no other changes,what is the effect of doubling only the concentration of the nucleophile in the above reaction?
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
50
Rank the species below in order of increasing nucleophilicity in protic solvents:
CH3CO2- ,CH3S- ,HO- ,H2O.
CH3CO2- ,CH3S- ,HO- ,H2O.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
51
Identify the alkyl halide that reacts the fastest in an SN1 reaction.
A)2-chloro-2-methylpropane
B)2-chlorobutane
C)1-chlorobutane
D)chloromethane
A)2-chloro-2-methylpropane
B)2-chlorobutane
C)1-chlorobutane
D)chloromethane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
52
Rank the species below in order of leaving group capabilities in SN2 reactions (worst leaving group to best):
CH3O-,H2O,C6H5SO3-,H2N-.
CH3O-,H2O,C6H5SO3-,H2N-.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
53
Assuming no other changes,what is the effect of doubling only the concentration of the alkyl halide in the above SN1 reaction?
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of the following correctly describes the relative nucleophilicities of methoxide and tert-butoxide?
A)These alkoxides have essentially the same nucleophilicities since the negative charge in both is localized on an oxygen atom.
B)Methoxide is more nucleophilic because the nucleophilicity of tert-butoxide is diminished by steric effects.
C)tert-Butoxide is more nucleophilic because it contains three methyl groups which increase the charge on its oxygen by donating electron density.
D)tert-Butoxide is more nucleophilic because it is more basic.
E)none of the above
A)These alkoxides have essentially the same nucleophilicities since the negative charge in both is localized on an oxygen atom.
B)Methoxide is more nucleophilic because the nucleophilicity of tert-butoxide is diminished by steric effects.
C)tert-Butoxide is more nucleophilic because it contains three methyl groups which increase the charge on its oxygen by donating electron density.
D)tert-Butoxide is more nucleophilic because it is more basic.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
55
Give the mechanism of the reaction shown below. 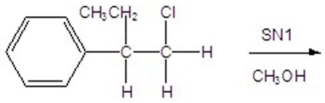


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
56
Assuming no other changes,what is the effect of doubling both the concentration of the alkyl halide and the nucleophile in the above reaction?
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved
A)no change
B)doubles the rate
C)triples the rate
D)quadruples the rate
E)rate is halved

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following iodides undergoes SN2 reaction with cyanide (CN-)the fastest?
A)1-iodo-3-methylpentane
B)2-iodopentane
C)2-iodo-2-methylpentane
D)3-iodopentane
E)1-iodo-2,2-dimethylpentane
A)1-iodo-3-methylpentane
B)2-iodopentane
C)2-iodo-2-methylpentane
D)3-iodopentane
E)1-iodo-2,2-dimethylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
58
Why,in a polar protic solvent,is iodide a better nucleophile than fluoride?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which halide reacts most rapidly via an SN2 mechanism?
A)(CH3)CCH2CH2F
B)(CH3)CCH2CH2Cl
C)(CH3)CCH2CH2Br
D)(CH3)CCH2CH2I
E)All primary halides react at the same rate in SN2.
A)(CH3)CCH2CH2F
B)(CH3)CCH2CH2Cl
C)(CH3)CCH2CH2Br
D)(CH3)CCH2CH2I
E)All primary halides react at the same rate in SN2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which halide reacts most rapidly via an SN2 mechanism?
A)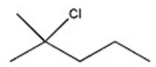
B)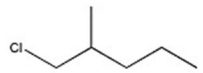
C)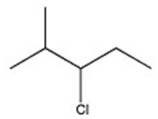
D)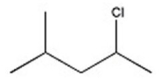
E)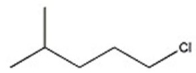
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following is the rate law for SN1 mechanisms?
A)Rate = k[Alkyl Halide] [Nucleophile]
B)Rate = k[Nucleophile]
C)Rate = k[Alkyl Halide]
D)Rate = k[Alkyl Halide] [Nucleophile] + k2[Alkyl Halide]
E)Rate = k1[Alkyl Halide] + k2[Nucleophile]
A)Rate = k[Alkyl Halide] [Nucleophile]
B)Rate = k[Nucleophile]
C)Rate = k[Alkyl Halide]
D)Rate = k[Alkyl Halide] [Nucleophile] + k2[Alkyl Halide]
E)Rate = k1[Alkyl Halide] + k2[Nucleophile]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
62
Provide the major organic product(s)of the reaction shown. 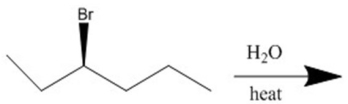


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following factors has no effect on the rate of SN1 reactions?
A)the nature of the alkyl halide
B)the nature of the leaving group
C)the concentration of the alkyl halide
D)the concentration of the nucleophile
E)the value of the rate constant
A)the nature of the alkyl halide
B)the nature of the leaving group
C)the concentration of the alkyl halide
D)the concentration of the nucleophile
E)the value of the rate constant

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
64
Provide the structure of the major organic product of the following reaction. 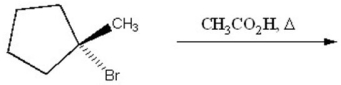


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
65
Provide the major organic product(s)in the reaction below. 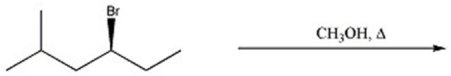
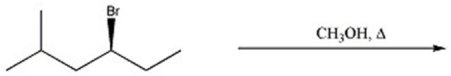

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
66
Provide the major organic products(s)of the reaction shown. 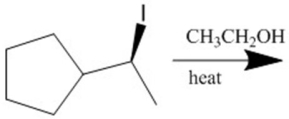


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
67
Provide the major organic product of the reaction below and a detailed,stepwise mechanism which accounts for its formation. 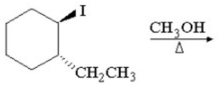
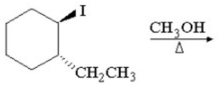

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which of the following carbocations is the least stable? 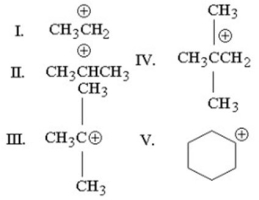
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
69
Provide the major organic product(s)in the reaction below. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
70
Provide the structure of the major organic products which result in the reaction below. 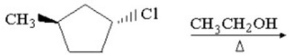
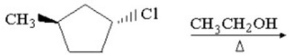

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the compounds below undergoes solvolysis in aqueous ethanol most rapidly?
A)cyclohexyl bromide
B)methyl iodide
C)isopropyl chloride
D)3-chloropentane
E)3-iodo-3-methylpentane
A)cyclohexyl bromide
B)methyl iodide
C)isopropyl chloride
D)3-chloropentane
E)3-iodo-3-methylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
72
In the SN1 hydrolysis mechanism of (CH3)3CBr,there are ________ elementary steps,________ distinct transition states,and ________ distinct intermediates.
A)2,2,2
B)2,2,3
C)2,3,2
D)3,2,3
E)3,3,2
A)2,2,2
B)2,2,3
C)2,3,2
D)3,2,3
E)3,3,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
73
The specific rotation of optically pure (R)-sec-butyl alcohol is -13.52°.An optically pure sample of (R)-sec-butyl bromide was converted into the corresponding sec-butyl alcohol via an SN2 reaction.What is the specific rotation of the product,assuming 100% yield?
A)-13.52°
B)between 0° and -13.52°
C)between 0° and +13.52°
D)+13.52°
E)zero
A)-13.52°
B)between 0° and -13.52°
C)between 0° and +13.52°
D)+13.52°
E)zero

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following iodides undergoes SN1 solvolysis in water the fastest?
A)1-iodo-3-methylpentane
B)2-iodopentane
C)2-iodo-2-methylpentane
D)3-iodopentane
E)1-iodo-2,2-dimethylpentane
A)1-iodo-3-methylpentane
B)2-iodopentane
C)2-iodo-2-methylpentane
D)3-iodopentane
E)1-iodo-2,2-dimethylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of the following alkyl halides gives the fastest SN1 reaction?
A)CH3CH2CH2Br
B)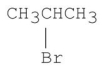
C)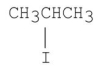
D)CH3CH2CH2I
E)CH3CH2CH2Cl
A)CH3CH2CH2Br
B)
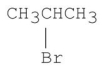
C)
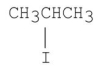
D)CH3CH2CH2I
E)CH3CH2CH2Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following alkyl halides gives the fastest SN1 reaction?
A)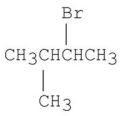
B)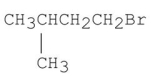
C)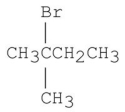
D)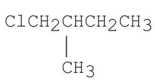
E)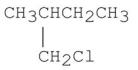
A)

B)
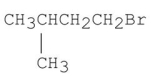
C)

D)

E)
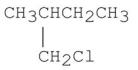

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
77
When (S)-2-bromobutane undergoes an SN2 reaction with CH3O-,the product is the compound shown below.What is/are the configuration(s)of the product(s)obtained from this reaction? 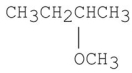
A)S only
B)R only
C)a mixture of enantiomers with more R than S
D)a mixture of enantiomers with more S than R
E)equal mixture of R and S
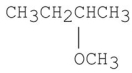
A)S only
B)R only
C)a mixture of enantiomers with more R than S
D)a mixture of enantiomers with more S than R
E)equal mixture of R and S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following carbocations is the most stable?
A)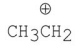
B)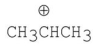
C)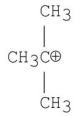
D)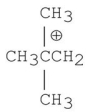
E)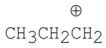
A)
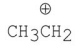
B)
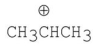
C)

D)

E)
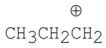

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
79
SN1 reactions usually proceed with
A)equal amounts of inversion and retention at the center undergoing substitution.
B)slightly more inversion than retention at the center undergoing substitution.
C)slightly more retention than inversion at the center undergoing substitution.
D)complete inversion at the center undergoing substitution.
E)complete retention at the center undergoing substitution.
A)equal amounts of inversion and retention at the center undergoing substitution.
B)slightly more inversion than retention at the center undergoing substitution.
C)slightly more retention than inversion at the center undergoing substitution.
D)complete inversion at the center undergoing substitution.
E)complete retention at the center undergoing substitution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck
80
Provide the major organic products(s)of the reaction shown. 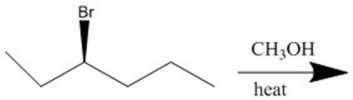


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 228 في هذه المجموعة.
فتح الحزمة
k this deck








