Deck 20: The Organic Chemistry of Carbohydrates
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/110
العب
ملء الشاشة (f)
Deck 20: The Organic Chemistry of Carbohydrates
1
Which of the following best describes the sugar D-galactose? 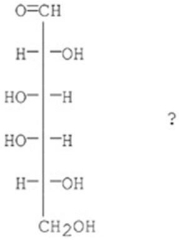
A)D-aldohexose
B)D-ketohexose
C)L-aldohexose
D)L-ketohexose
E)D-aldopentose

A)D-aldohexose
B)D-ketohexose
C)L-aldohexose
D)L-ketohexose
E)D-aldopentose
D-aldohexose
2
At which carbon are the following sugars epimers of each other? 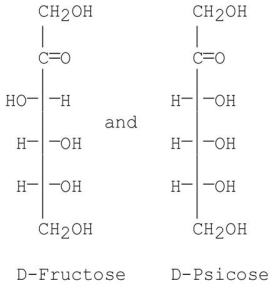
A)C-1
B)C-2
C)C-3
D)C-4
E)C-5

A)C-1
B)C-2
C)C-3
D)C-4
E)C-5
C-3
3
The open-chain form of D-idose is shown below.Draw the open-chain form of the C-3 epimer of D-idose. 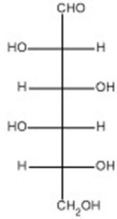

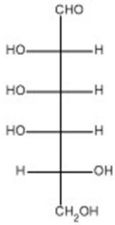
4
Which of the following compounds is a D-sugar?
A)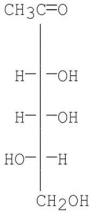
B)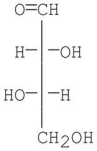
C)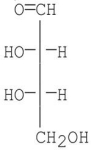
D)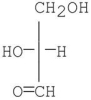
E)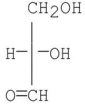
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
5
Draw L-erythrose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
6
All chiral D-sugars rotate plane-polarized light
A)clockwise.
B)counterclockwise.
C)+20.0°.
D)in a direction that cannot be predicted but must be determined experimentally.
E)since they are optically inactive.
A)clockwise.
B)counterclockwise.
C)+20.0°.
D)in a direction that cannot be predicted but must be determined experimentally.
E)since they are optically inactive.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following corresponds to the definition of an aldopentose? I.It is a monosaccharide.
II)It contains a CHO group
III)It is a disaccharide.
IV)It is an oligosaccharide.
A)I and II
B)II and III
C)I and IV
D)I and III
E)I,II,and III
II)It contains a CHO group
III)It is a disaccharide.
IV)It is an oligosaccharide.
A)I and II
B)II and III
C)I and IV
D)I and III
E)I,II,and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
8
Draw the Fischer projection for the open-chain form of D-glucose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
9
At which carbon are the following sugars epimers of each other? 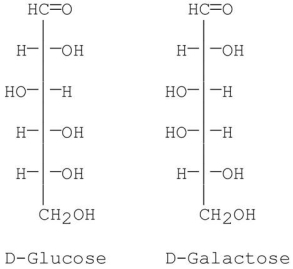
A)C-1
B)C-2
C)C-3
D)C-4
E)C-5

A)C-1
B)C-2
C)C-3
D)C-4
E)C-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the relationship between the following compounds? 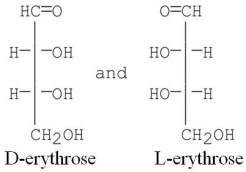
A)conformational isomers
B)constitutional isomers
C)identical
D)enantiomers
E)diastereomers

A)conformational isomers
B)constitutional isomers
C)identical
D)enantiomers
E)diastereomers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many chirality centers are there in an aldohexose?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
12
A carbohydrate composed of three to ten sugar molecules is called a(n)
A)single carbohydrate.
B)disaccharide.
C)oligosaccharide.
D)polysaccharide.
E)monosaccharide.
A)single carbohydrate.
B)disaccharide.
C)oligosaccharide.
D)polysaccharide.
E)monosaccharide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many chirality centers are there in a 2-ketohexose?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
14
At which carbon are the following sugars epimers of each other? 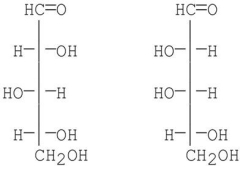
A)C-1
B)C-2
C)C-3
D)C-4
E)C-5

A)C-1
B)C-2
C)C-3
D)C-4
E)C-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
15
Draw the Fischer projection for the open-chain form of D-erythrose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
16
Assign the proper configurational descriptor to each asymmetric center in D-glucose below. 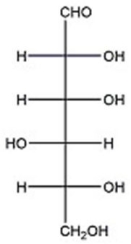


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
17
How many stereoisomers are possible for a 2-ketohexose?
A)2
B)4
C)8
D)16
E)32
A)2
B)4
C)8
D)16
E)32

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
18
Is the following structure of glucose a D or L configuration? Explain. 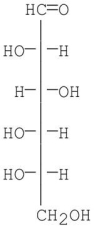


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following statements best describes the difference between amylose and amylopectin?
A)Amylose is a branched polysaccharide while amylopectin is a chain polysaccharide.
B)Amylose is a straight-chain polysaccharide while amylopectin is a branched polysaccharide.
C)Amylose contains α-1,6-glycosidic linkage which amylopectin does not contain.
D)Amylose is composed of thousands of D-glucose units while amylopectin is composed of thousands of D-galactose units.
E)Amylose is one of the largest molecules found in nature while amylopectin is one of the smallest molecules found in nature.
A)Amylose is a branched polysaccharide while amylopectin is a chain polysaccharide.
B)Amylose is a straight-chain polysaccharide while amylopectin is a branched polysaccharide.
C)Amylose contains α-1,6-glycosidic linkage which amylopectin does not contain.
D)Amylose is composed of thousands of D-glucose units while amylopectin is composed of thousands of D-galactose units.
E)Amylose is one of the largest molecules found in nature while amylopectin is one of the smallest molecules found in nature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
20
Is L-glucose the enantiomer of D-glucose,the C-5 epimer of D-glucose,or both?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
21
Give the major product(s)for the reaction.You may choose more than one answer. 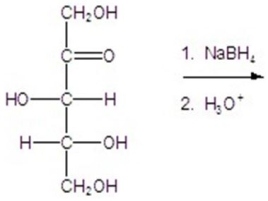
A)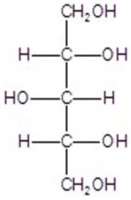
B)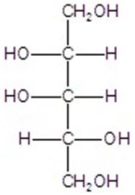
C)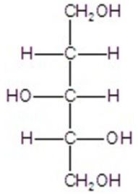
D)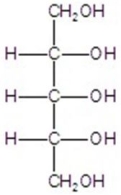
E)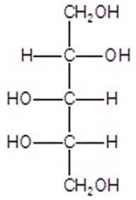

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
22
Provide the Fischer projection of the open-chain form of the aldonic acid which results when L-glucose is treated with bromine water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
23
An optically active D-aldopentose produced an optically active alditol upon treatment with NaBH4.When this aldopentose was subjected to a Ruff degradation,a D-aldotetrose was generated.This aldotetrose gave an optically active aldaric acid upon oxidation with HNO3.Use these data to provide the structure of the starting D-aldopentose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
24
Provide the major organic product(s)of the reaction below. 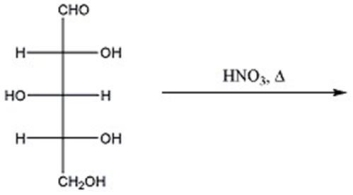


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the D-aldopentoses yields an optically inactive aldonic acid upon oxidation with bromine water? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
26
An optically active D-aldopentose produced an optically inactive aldaric acid upon treatment with HNO3.When this aldopentose was subjected to a Ruff degradation,a D-aldotetrose was generated.This aldotetrose gave an optically inactive alditol upon reduction with NaBH4.Use these data to provide the structure of the starting D-aldopentose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
27
An optically active D-aldopentose A produced an optically active alditol B upon treatment with sodium borohydride.When this aldopentose was subjected to a Ruff degradation,a D-aldotetrose C was generated.This aldotetrose yielded an optically inactive aldaric acid D upon oxidation with nitric acid.Use these data to provide the structures of compounds A,B,C,and D.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
28
Provide the major organic product(s)of the reaction below. 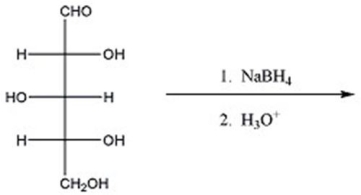


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
29
Name the following compound. 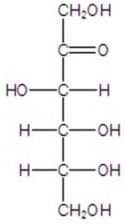
A)D-glucose
B)D-galactose
C)D-fructose
D)D-arabinose
E)D-talose

A)D-glucose
B)D-galactose
C)D-fructose
D)D-arabinose
E)D-talose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following sugars yields the same alditol upon reduction with NaBH4 as does D-glucose?
A)D-fructose
B)D-mannose
C)L-glucose
D)D-arabinose
E)none of the above
A)D-fructose
B)D-mannose
C)L-glucose
D)D-arabinose
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following monosaccharides will form D-glucaric acid upon oxidation with nitric acid? 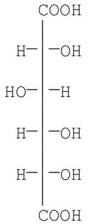
A)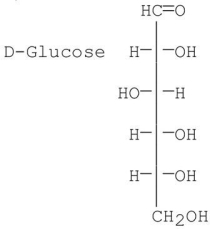
B)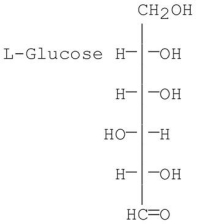
C)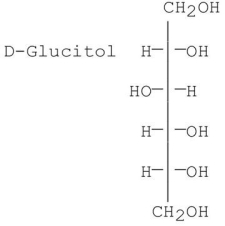
D)A and B
E)A and C

A)

B)

C)

D)A and B
E)A and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
32
Provide the structure of the product which results when D-ribose is treated with bromine water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following is/are the product(s)obtained from the reduction of D-fructose?
A)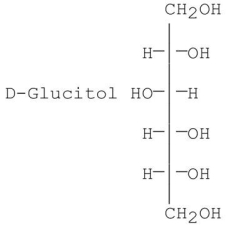
B)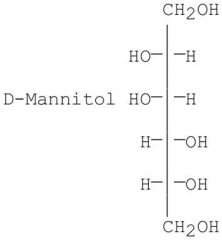
C)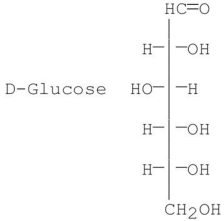
D)A and B
E)A and C
A)

B)

C)

D)A and B
E)A and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
34
Reduction of a 2-ketohexose with NaBH4 yields
A)a single aldohexose.
B)a mixture of acetals.
C)a mixture of alditols.
D)a mixture of cyclic hemiacetals.
E)a single pyranose.
A)a single aldohexose.
B)a mixture of acetals.
C)a mixture of alditols.
D)a mixture of cyclic hemiacetals.
E)a single pyranose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
35
An optically active D-aldopentose produced an optically inactive alditol upon treatment with NaBH4.When this aldopentose was subjected to a Ruff degradation,a D-aldotetrose was generated.This aldotetrose gave an optically active aldaric acid upon oxidation with HNO3.Use these data to provide the structure of the starting D-aldopentose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
36
When D-sorbose (shown below)is treated with NaBH4,which of the following is produced? 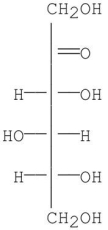
A)a single optically active alditol
B)a single optically inactive alditol
C)a racemic mixture of alditols
D)an optically inactive mixture of epimeric alditols
E)an optically active mixture of epimeric alditols

A)a single optically active alditol
B)a single optically inactive alditol
C)a racemic mixture of alditols
D)an optically inactive mixture of epimeric alditols
E)an optically active mixture of epimeric alditols

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
37
Draw the Fischer projection for the open-chain form of D-fructose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
38
When D-tagatose is added to a basic aqueous solution,an equilibrium mixture of three monosaccharides is obtained.Identify the monosaccharides. 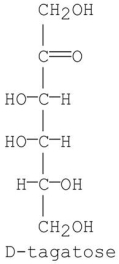


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
39
When D-threose is treated with NaBH4
A)a 70:30 mixture of enantiomeric alditols results.
B)a 50:50 mixture of enantiomeric alditols results.
C)a meso alditol is produced.
D)the product mixture contains two diastereomeric alditols.
E)an optically active alditol is produced.
A)a 70:30 mixture of enantiomeric alditols results.
B)a 50:50 mixture of enantiomeric alditols results.
C)a meso alditol is produced.
D)the product mixture contains two diastereomeric alditols.
E)an optically active alditol is produced.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
40
Give the major product(s)for the reaction. 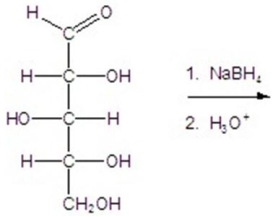
A)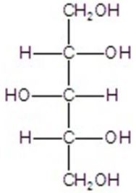
B)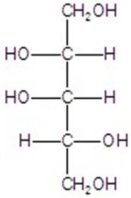
C)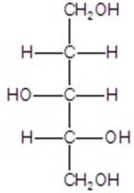
D)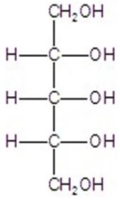
E)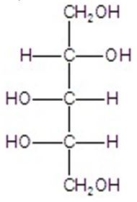

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
41
Give the product(s)for each step of the following reaction. 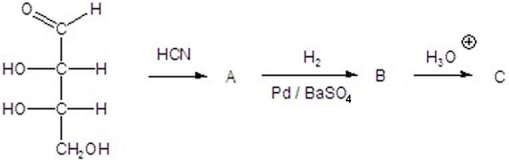


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
42
Provide the open-chair structures of the two monosaccharides that result when D-threose undergoes the Kiliani-Fischer synthesis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
43
What nucleophilic carbon species is used in the Kiliani-Fischer synthesis?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
44
Give the major product(s)for the following reaction. 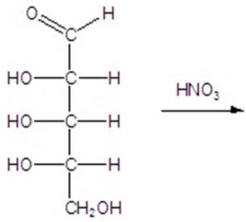
A)no reaction
B)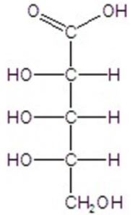
C)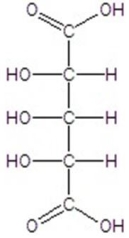
D)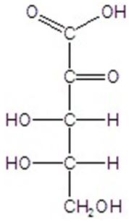
E)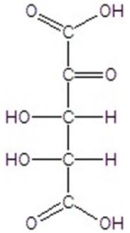

A)no reaction
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following is the Haworth projection of a α-D-tagatofuranose? 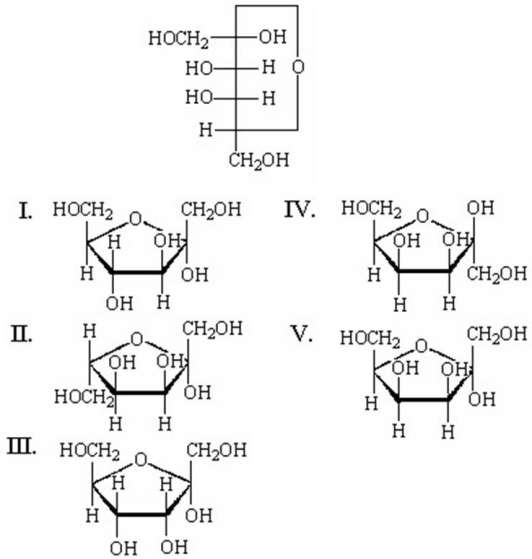
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
46
Give the product(s)for each step of the following reaction. 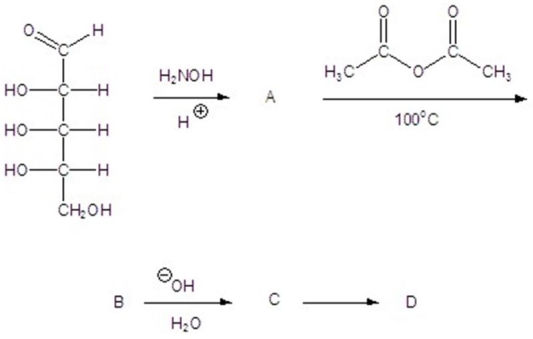


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
47
Two D-aldopentoses give the same D-aldotetrose upon Ruff degradation.The two aldopentoses are
A)enantiomers.
B)meso compounds.
C)C2 epimers.
D)C4 epimers.
E)D-glucose and D-galactose.
A)enantiomers.
B)meso compounds.
C)C2 epimers.
D)C4 epimers.
E)D-glucose and D-galactose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following is the Haworth projection of β-D-galactopyranose? 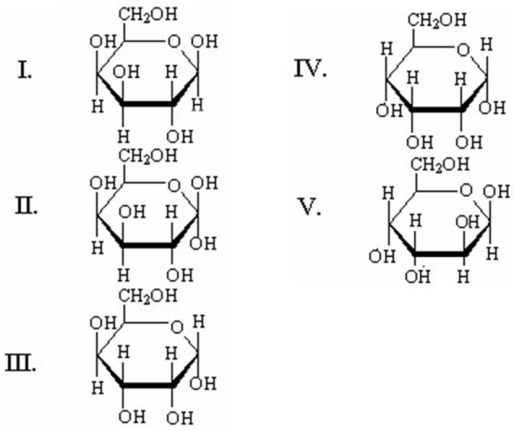
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
49
Give the major product(s)for the following reaction. 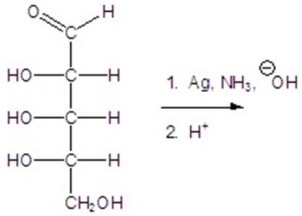
A)no reaction
B)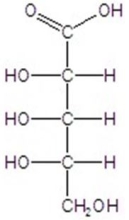
C)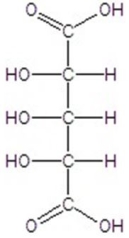
D)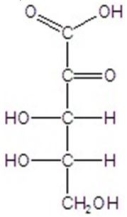
E)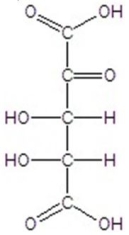

A)no reaction
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following statements best describes the meaning of mutorotation?
A)a rapid exchange between the α and β forms of diasternomeric sugars
B)a rapid exchange between the D and L forms
C)a slow exchange between hydrogen and deuterated hydrogen
D)a slow change in optical rotation to reach an equilibrium value
E)a slow change in absolute configuration to reach an equilibrium value
A)a rapid exchange between the α and β forms of diasternomeric sugars
B)a rapid exchange between the D and L forms
C)a slow exchange between hydrogen and deuterated hydrogen
D)a slow change in optical rotation to reach an equilibrium value
E)a slow change in absolute configuration to reach an equilibrium value

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following sequences is known as a Kiliani-Fischer chain elongation?
A)1)Fe3+,H2O2 2.Br2,H2O 3.Ca(OH)2
B)1)CN- 2.H2,Pd/BaSO4 3.H3O+
C)1)NaBH4 2.H2O 3.Br2,H2O
D)1)acetic anhydride (excess),pyridine 2.CH3I (excess),Ag2O
E)1)CH3OH,H+,Δ 2.AgNO3,Δ
A)1)Fe3+,H2O2 2.Br2,H2O 3.Ca(OH)2
B)1)CN- 2.H2,Pd/BaSO4 3.H3O+
C)1)NaBH4 2.H2O 3.Br2,H2O
D)1)acetic anhydride (excess),pyridine 2.CH3I (excess),Ag2O
E)1)CH3OH,H+,Δ 2.AgNO3,Δ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
52
Give the major product(s)for the following reaction. 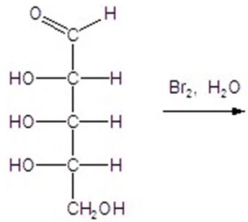
A)no reaction
B)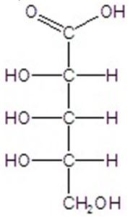
C)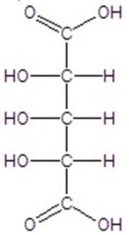
D)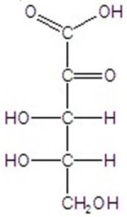
E)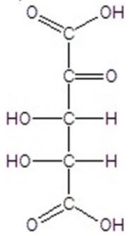

A)no reaction
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
53
In solution,glucose exists as
A)the open-chain form only.
B)the cyclic hemiacetal form only.
C)the cyclic acetal form only.
D)an equilibrium mixture of the open-chain form and cyclic acetal forms.
E)an equilibrium mixture of the open-chain form and cyclic hemiacetal forms.
A)the open-chain form only.
B)the cyclic hemiacetal form only.
C)the cyclic acetal form only.
D)an equilibrium mixture of the open-chain form and cyclic acetal forms.
E)an equilibrium mixture of the open-chain form and cyclic hemiacetal forms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which monosaccharides are formed in the Kiliani-Fischer synthesis starting with D-xylose? 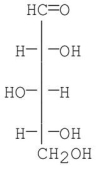
A)D-glucose and D-mannose
B)D-glucose and D-idose
C)D-galactose and D-talose
D)D-allose and D-altrose
E)D-ribose and D-arabinose

A)D-glucose and D-mannose
B)D-glucose and D-idose
C)D-galactose and D-talose
D)D-allose and D-altrose
E)D-ribose and D-arabinose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
55
Give the major product(s)for the following reaction. 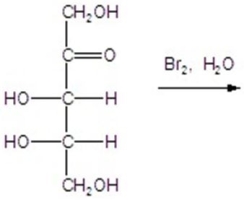
A)no reaction
B)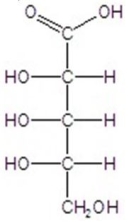
C)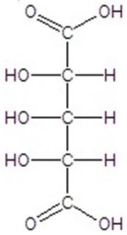
D)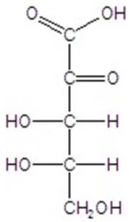
E)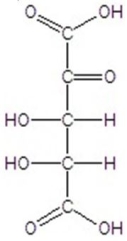

A)no reaction
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which two monosaccharides can be degraded by the Ruff Degradation to D-arabinose? 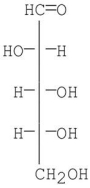
A)D-allose and D-altrose
B)D-glucose and D-idose
C)D-galactose and D-talose
D)D-erythrose and D-threose
E)D-glucose and D-mannose

A)D-allose and D-altrose
B)D-glucose and D-idose
C)D-galactose and D-talose
D)D-erythrose and D-threose
E)D-glucose and D-mannose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following is the cyclic hemiacetal formed from 4-hydroxyheptanal? 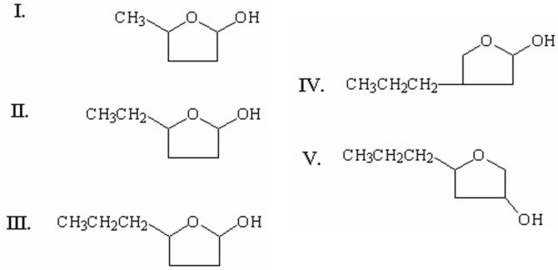
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
58
Give the major product(s)for the following reaction. 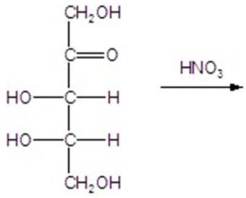
A)no reaction
B)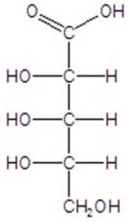
C)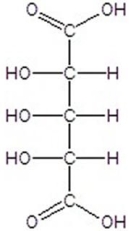
D)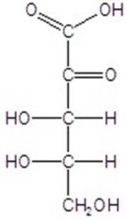
E)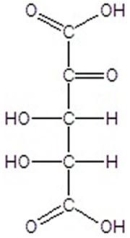

A)no reaction
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
59
When a monosaccharide reacts to give the pyranose form from its open-chain form,how many distinct pyranose forms are possible?
A)1
B)2
C)2n,where n is the number of carbons present
D)4n + 2,where n is the number of carbons present
E)4
A)1
B)2
C)2n,where n is the number of carbons present
D)4n + 2,where n is the number of carbons present
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
60
An aqueous solution of glucose behaves as an aldehyde because
A)it is hydrolyzed by water to the free aldehyde.
B)it is a ketone,but is in equilibrium with the aldehyde form.
C)glucose is actually a cyclic aldehyde.
D)its cyclic hemiacetal,the predominant form,is in equilibrium with the free aldehyde form.
E)it can be oxidized with periodic acid.
A)it is hydrolyzed by water to the free aldehyde.
B)it is a ketone,but is in equilibrium with the aldehyde form.
C)glucose is actually a cyclic aldehyde.
D)its cyclic hemiacetal,the predominant form,is in equilibrium with the free aldehyde form.
E)it can be oxidized with periodic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
61
Draw the Haworth structure of α-D-ribofuranose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
62
Six-membered cyclic hemiacetals and five-membered cyclic hemiacetals are called,respectively
A)mannoses and xyloses.
B)maltoses and arabinoses.
C)pyranoses and fructoses.
D)glycoses and fructoses.
E)none of the above.
A)mannoses and xyloses.
B)maltoses and arabinoses.
C)pyranoses and fructoses.
D)glycoses and fructoses.
E)none of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
63
The open-chain form of D-idose is shown below.Draw the Haworth projection of a-D-idopyranose. 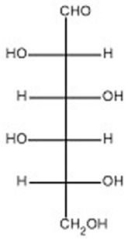


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
64
Given the structure of D-altrose below,provide the Haworth structure of β-D-altropyranose. 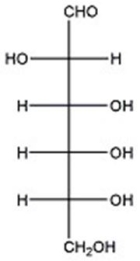


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
65
Provide the Haworth projection of ethyl-β-D-mannopyranoside.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
66
Draw the Haworth structure of β-D-glucopyranose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
67
A pyranose with the hydroxyl group on the anomeric carbon pointing up in the Haworth structure is designated
A)a'.
B)b'.
C)α)
D)β)
E)f)
A)a'.
B)b'.
C)α)
D)β)
E)f)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
68
Why is β-D-glucopyranose more stable in nature than α-D-glucopyranose?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following is methyl-α-D-glucopyranoside? 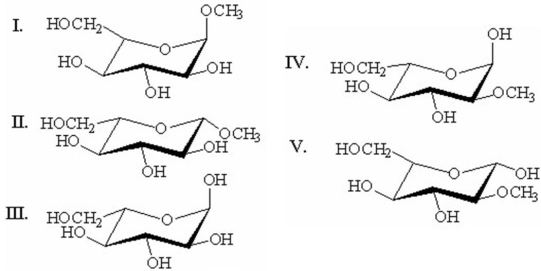
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
70
Draw the more stable chair conformer of α-D-glucopyranose.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
71
Anomers of D-glucopyranose differ in their stereochemistry at
A)C1.
B)C2.
C)C3.
D)C4.
E)C5.
A)C1.
B)C2.
C)C3.
D)C4.
E)C5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the following statements best describes the meaning of a glycoside?
A)It is the mirror image of a sugar.
B)It is the hemiacetal of a sugar.
C)It is the acetal of a sugar.
D)It is the enantiomer of a sugar.
E)It is the enol-keto form of a sugar.
A)It is the mirror image of a sugar.
B)It is the hemiacetal of a sugar.
C)It is the acetal of a sugar.
D)It is the enantiomer of a sugar.
E)It is the enol-keto form of a sugar.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following statements regarding methyl-β-L-glucopyranoside is correct?
A)This glycoside is a reducing sugar.
B)This glycoside undergoes mutorotation in aqueous base.
C)This glycoside will undergo no reaction when treated with excess CH3I/Ag2O.
D)This glycoside will be hydrolyzed to the cyclic hemiacetal in dilute aqueous acid.
E)This glycoside will readily undergo Kiliani-Fischer chain elongation.
A)This glycoside is a reducing sugar.
B)This glycoside undergoes mutorotation in aqueous base.
C)This glycoside will undergo no reaction when treated with excess CH3I/Ag2O.
D)This glycoside will be hydrolyzed to the cyclic hemiacetal in dilute aqueous acid.
E)This glycoside will readily undergo Kiliani-Fischer chain elongation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
74
Under what conditions is the methyl glycoside of galactose prepared?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
75
When pure α-D-glucopyranose is dissolved in water,the optical rotation of the resulting solution changes over a period of time.What is the name of this phenomenon and why does it occur?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
76
The open-chain form of D-idose is shown below.Draw the Haworth projection of b-D-idopyranose. 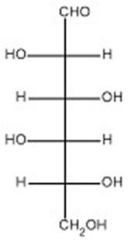


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
77
Given the structure of D-arabinose below,provide the structure of the glycoside methyl α-D-arabinofuranoside. 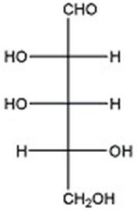


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following compounds is a nonreducing sugar? 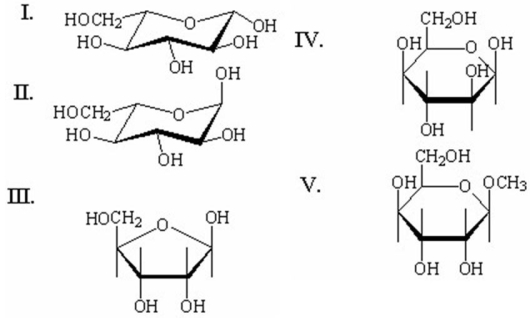
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
79
Give the name for the following structure. 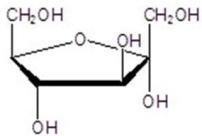


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck
80
Given the structure of D-talose below,provide the Haworth structure of α-D-talopyranose. 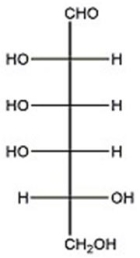


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 110 في هذه المجموعة.
فتح الحزمة
k this deck








