Deck 13: The Properties of Solutions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/73
العب
ملء الشاشة (f)
Deck 13: The Properties of Solutions
1
Cadmium bromide is used in photography and lithography.Calculate the molality of a solution prepared by dissolving 45.38 g of CdBr2 in 375.0 g of water.
A)0.03035 m
B)0.01600 m
C)0.1210 m
D)0.4446 m
E)None of these choices is correct.
A)0.03035 m
B)0.01600 m
C)0.1210 m
D)0.4446 m
E)None of these choices is correct.
0.4446 m
2
A solution of sucrose (sugar)in water is in equilibrium with solid sucrose.If more solid sucrose is now added,with stirring,
A)the concentration of the solution will increase.
B)the concentration of the solution will decrease.
C)the concentration of the solution will remain the same.
D)the volume of solution will increase.
E)a supersaturated solution will be produced.
A)the concentration of the solution will increase.
B)the concentration of the solution will decrease.
C)the concentration of the solution will remain the same.
D)the volume of solution will increase.
E)a supersaturated solution will be produced.
the concentration of the solution will remain the same.
3
The solubility of the oxidizing agent potassium permanganate is 7.1 g per 100.0 g of water at 25°C.What is the mole fraction of potassium permanganate in this solution?
A)0.0080
B)0.0086
C)0.066
D)0.45
E)0.48
A)0.0080
B)0.0086
C)0.066
D)0.45
E)0.48
0.0080
4
Isoamyl salicylate ( 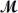
= 208.25 g/mol)has a pleasant aroma and is used in perfumes and soaps.Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
A)117.2 g isoamyl salicylate in 950.0 mL of ethyl alcohol
B)117.2 g isoamyl salicylate in 750.0 mL of ethyl alcohol
C)117.2 g isoamyl salicylate in 750.0 mL of solution
D)117.2 g isoamyl salicylate in 592.0 g of ethyl alcohol
E)None of these choices is correct.
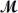
= 208.25 g/mol)has a pleasant aroma and is used in perfumes and soaps.Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
A)117.2 g isoamyl salicylate in 950.0 mL of ethyl alcohol
B)117.2 g isoamyl salicylate in 750.0 mL of ethyl alcohol
C)117.2 g isoamyl salicylate in 750.0 mL of solution
D)117.2 g isoamyl salicylate in 592.0 g of ethyl alcohol
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 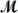
urea = 60.06 g/mol
A)Dissolve 120 g of urea in 1.00 kg of distilled water.
B)Dissolve 120 g of urea in 880 g of distilled water.
C)Dissolve 120 g of urea in enough distilled water to produce 1.00 L of solution.
D)Dissolve 120 g of urea in 1.00 liter of distilled water.
E)The density of urea is needed in order to do this calculation.
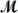
urea = 60.06 g/mol
A)Dissolve 120 g of urea in 1.00 kg of distilled water.
B)Dissolve 120 g of urea in 880 g of distilled water.
C)Dissolve 120 g of urea in enough distilled water to produce 1.00 L of solution.
D)Dissolve 120 g of urea in 1.00 liter of distilled water.
E)The density of urea is needed in order to do this calculation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
6
When two pure substances are mixed to form a solution
A)heat is released.
B)heat is absorbed.
C)there is an increase in entropy.
D)there is a decrease in entropy.
E)entropy is conserved.
A)heat is released.
B)heat is absorbed.
C)there is an increase in entropy.
D)there is a decrease in entropy.
E)entropy is conserved.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the molality of a solution prepared by dissolving 86.9 g of diethyl ether,C4H10O,in 425 g of benzene,C6H6?
A)0.362 m
B)0.498 m
C)2.01 m
D)2.76 m
E)None of these choices is correct.
A)0.362 m
B)0.498 m
C)2.01 m
D)2.76 m
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following sets of conditions could exist when two liquids which are completely miscible in one another are mixed?
A)( Hsoln > 0,entropy of system decreases)
B)( Hsoln 0,entropy of system decreases)
C)( Hsoln 0,entropy change of system 0)
D)( Hsoln 0,entropy of system increases)
E)None of these choices is correct.
A)( Hsoln > 0,entropy of system decreases)
B)( Hsoln 0,entropy of system decreases)
C)( Hsoln 0,entropy change of system 0)
D)( Hsoln 0,entropy of system increases)
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
9
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers.Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution.
A)0.732 M
B)0.865 M
C)2.66 M
D)4.97 M
E)None of these choices is correct.
A)0.732 M
B)0.865 M
C)2.66 M
D)4.97 M
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
10
For a given solution,which of the following concentration values will change as temperature changes?
A)mass percent
B)molality
C)mole fraction
D)molarity
E)None of these choices is correct.
A)mass percent
B)molality
C)mole fraction
D)molarity
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
11
Potassium fluoride is used for frosting glass.Calculate the molarity of a solution prepared by dissolving 78.6 g of KF in enough water to produce 225 mL of solution.
A)0.304 M
B)0.349 M
C)1.35 M
D)3.29 M
E)6.01 M
A)0.304 M
B)0.349 M
C)1.35 M
D)3.29 M
E)6.01 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
12
The Henry's Law constant (k)for carbon monoxide in water at 25°C is 9.71 10-4 mol/(L·atm).How many grams of CO will dissolve in 1.00 L of water if the partial pressure of CO is 2.75 atm?
A)3.53 10-4 g
B)2.67 10-3 g
C)9.89 10-3 g
D)7.48 10-2 g
E)None of these choices is correct.
A)3.53 10-4 g
B)2.67 10-3 g
C)9.89 10-3 g
D)7.48 10-2 g
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
13
Saccharin,one of the first non-nutritive sweeteners used in soft-drinks,is 500 times sweeter than sugar in dilute aqueous solutions.The solubility of saccharin is 1.00 gram per 290 mL of solution.What is the molarity of a saturated saccharin solution? 
saccharin= 183.2 g/mol
A)0.0188 M
B)0.632 M
C)1.58 M
D)3.45 M
E)None of these choices is correct.

saccharin= 183.2 g/mol
A)0.0188 M
B)0.632 M
C)1.58 M
D)3.45 M
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
14
Calculate the molarity of a solution prepared by diluting 1.85 L of 6.5 M KOH to 11.0 L.
A)0.28 M
B)0.91 M
C)1.1 M
D)3.1 M
E)3.9 M
A)0.28 M
B)0.91 M
C)1.1 M
D)3.1 M
E)3.9 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
15
If a solute dissolves in an endothermic process
A)H bonds must exist between solvent and solute.
B)strong ion-dipole forces must exist in the solution.
C)the solute must be a gas.
D)the entropy of the solution is immaterial.
E)the entropy of the solution must be greater than that of its pure components.
A)H bonds must exist between solvent and solute.
B)strong ion-dipole forces must exist in the solution.
C)the solute must be a gas.
D)the entropy of the solution is immaterial.
E)the entropy of the solution must be greater than that of its pure components.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
16
The chemist,Anna Lytic,must prepare 1.00 kg of 15.0% (w/w)acetic acid using a stock solution which is 36.0% (w/w)acetic acid (d = 1.045 g/mL).Which of the following combinations will give her the solution she wants?
A)417 mL of 36% acetic acid in 583 mL of distilled water
B)417 g of 36% acetic acid in 583 g of distilled water
C)360 mL of 36% acetic acid in 640 mL of distilled water
D)360 g of 36% acetic acid in 640 g of distilled water
E)150 g of 36% acetic acid in 850 g of distilled water
A)417 mL of 36% acetic acid in 583 mL of distilled water
B)417 g of 36% acetic acid in 583 g of distilled water
C)360 mL of 36% acetic acid in 640 mL of distilled water
D)360 g of 36% acetic acid in 640 g of distilled water
E)150 g of 36% acetic acid in 850 g of distilled water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
17
Methane has a Henry's Law constant (k)of 9.88 10-2 mol/(L·atm)when dissolved in benzene at 25°C.How many grams of CH4 will dissolve in 3.00 L of benzene if the partial pressure of CH4 is 1.48 atm?
A)0.0667 g
B)0.146 g
C)2.34 g
D)4.83 g
E)7.02 g
A)0.0667 g
B)0.146 g
C)2.34 g
D)4.83 g
E)7.02 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
18
Copper(II)bromide is used as a wood preservative.What mass of CuBr2 is needed to prepare 750.0 mL of a 1.25 M solution?
A)134 g
B)209 g
C)372 g
D)938 g
E)> 1 kg
A)134 g
B)209 g
C)372 g
D)938 g
E)> 1 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
19
What volume of concentrated (14.7 M)phosphoric acid is needed to prepare 25.0 L of 3.0 M H3PO4?
A)0.20 L
B)0.57 L
C)1.8 L
D)3.6 L
E)5.1 L
A)0.20 L
B)0.57 L
C)1.8 L
D)3.6 L
E)5.1 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
20
Calcium nitrite is used as a corrosion inhibitor in lubricants.What is the molality of a solution prepared by dissolving 18.5 g of calcium nitrite in 83.5 g of distilled water?
A)0.0342 m
B)0.0855 m
C)0.222 m
D)0.444 m
E)1.68 m
A)0.0342 m
B)0.0855 m
C)0.222 m
D)0.444 m
E)1.68 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
21
The mole fraction of potassium nitrate in an aqueous solution is 0.0194.The solution's density is 1.0627 g/mL.Calculate the molarity of the solution.
A)0.0194 M
B)0.981 M
C)1.05 M
D)1.96 M
E)19.4 M
A)0.0194 M
B)0.981 M
C)1.05 M
D)1.96 M
E)19.4 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
22
Select the weakest electrolyte from the following set.
A)Na2SO4
B)KCl
C)CH3CH2COOH,propionic acid
D)CaCl2
E)LiOH
A)Na2SO4
B)KCl
C)CH3CH2COOH,propionic acid
D)CaCl2
E)LiOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
23
Colligative properties depend on
A)the chemical properties of the solute.
B)the chemical properties of the solvent.
C)the masses of the individual ions.
D)the molar mass of the solute.
E)the number of particles dissolved.
A)the chemical properties of the solute.
B)the chemical properties of the solvent.
C)the masses of the individual ions.
D)the molar mass of the solute.
E)the number of particles dissolved.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
24
From the following list of aqueous solutions and water,select the one with the highest boiling point.
A)1.0 M KNO3
B)0.75 M NaCl
C)0.75 M CuCl2
D)2.0 M C12H22O11 (sucrose)
E)pure water
A)1.0 M KNO3
B)0.75 M NaCl
C)0.75 M CuCl2
D)2.0 M C12H22O11 (sucrose)
E)pure water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
25
A 0.89% (w/v)sodium chloride solution is referred to as physiological saline solution because it has the same concentration of salts as human blood.What is the molarity of a physiological saline solution?
A)0.0028 M
B)0.015 M
C)0.15 M
D)0.30 M
E)0.35 M
A)0.0028 M
B)0.015 M
C)0.15 M
D)0.30 M
E)0.35 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many moles of sulfate ions are present in 1.0 L of 0.5 M Li2SO4?
A)0.5 mol
B)1.0 mol
C)1.5 mol
D)2.0 mol
E)3.0 mol
A)0.5 mol
B)1.0 mol
C)1.5 mol
D)2.0 mol
E)3.0 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
27
Raoult's Law relates the vapor pressure of the solvent above the solution to its mole fraction in the solution.Which of the following is an accurate statement?
A)Raoult's Law applies exactly to all solutions.
B)Raoult's Law works best when applied to concentrated solutions.
C)Raoult's Law works best when applied to dilute solutions.
D)Raoult's Law applies only to non-ideal solutions.
E)None of these statements is accurate.
A)Raoult's Law applies exactly to all solutions.
B)Raoult's Law works best when applied to concentrated solutions.
C)Raoult's Law works best when applied to dilute solutions.
D)Raoult's Law applies only to non-ideal solutions.
E)None of these statements is accurate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
28
Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl.Which of the following statements is true?
A)The Na2CO3 solution has a higher osmotic pressure and higher vapor pressure than the LiCl solution.
B)The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.
C)The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than the LiCI solution.
D)The Na2CO3 solution has a lower osmotic pressure and higher boiling point than the LiCl solution.
E)None of these statements is true.
A)The Na2CO3 solution has a higher osmotic pressure and higher vapor pressure than the LiCl solution.
B)The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.
C)The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than the LiCI solution.
D)The Na2CO3 solution has a lower osmotic pressure and higher boiling point than the LiCl solution.
E)None of these statements is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
29
A 2.0% (w/v)solution of sodium hydrogen citrate,Na2C6H6O7,which also contains 2.5% (w/v)of dextrose,C6H12O6,is used as an anticoagulant for blood which is to be used for transfusions.What is the molarity of the sodium hydrogen citrate in the solution?
A)0.085 M
B)0.19 M
C)0.53 M
D)1.2 M
E)1.3 M
A)0.085 M
B)0.19 M
C)0.53 M
D)1.2 M
E)1.3 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
30
From the following list of aqueous solutions and water,select the one with the lowest freezing point.
A)0.75 M (NH4)3PO4
B)l.0 M CaSO4
C)l 0 M LiClO4
D)1.5 M CH3OH,methyl alcohol
E)pure water
A)0.75 M (NH4)3PO4
B)l.0 M CaSO4
C)l 0 M LiClO4
D)1.5 M CH3OH,methyl alcohol
E)pure water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following aqueous solutions should demonstrate the most non-ideal behavior?
A)0.1 M NaI
B)0.2 M CuSO4
C)0.5 M KBr
D)2.0 M CuCl2
E)2.5 M CsCl
A)0.1 M NaI
B)0.2 M CuSO4
C)0.5 M KBr
D)2.0 M CuCl2
E)2.5 M CsCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
32
Select the strongest electrolyte from the following set.
A)CH3CH2OH,ethanol
B)LiNO3
C)C6H12O6,glucose
D)CCl4
E)HF
A)CH3CH2OH,ethanol
B)LiNO3
C)C6H12O6,glucose
D)CCl4
E)HF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following aqueous solutions should demonstrate the most ideal behavior?
A)0.1 M K2SO4
B)0.1 M CaCl2
C)3.0 M LiF
D)0.1 M MgSO4
E)0.1 M NaCl
A)0.1 M K2SO4
B)0.1 M CaCl2
C)3.0 M LiF
D)0.1 M MgSO4
E)0.1 M NaCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
34
The concentration of iodine in sea water is 60.parts per billion by mass.If one assumes that the iodine exists in the form of iodide anions,what is the molarity of iodide in sea water? (The density of sea water is 1.025 g/mL. )
A)4.8 10-13 M
B)4.8 10-10 M
C)4.8 10-7 M
D)4.7 10-4 M
E)4.7 10-1 M
A)4.8 10-13 M
B)4.8 10-10 M
C)4.8 10-7 M
D)4.7 10-4 M
E)4.7 10-1 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many moles of solute particles are present in 100.0 mL of 2.50 M (NH4)3PO4?
A)0.100 mol
B)0.250 mol
C)0.500 mol
D)0.750 mol
E)l.00 mol
A)0.100 mol
B)0.250 mol
C)0.500 mol
D)0.750 mol
E)l.00 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
36
Sodium hydroxide is a common ingredient in drain cleaners such as Drano.The mole fraction of sodium hydroxide in a saturated aqueous solution is 0.310.What is the molality of the solution?
A)0.310 m
B)0.690 m
C)1.24 m
D)12.4 m
E)25.0 m
A)0.310 m
B)0.690 m
C)1.24 m
D)12.4 m
E)25.0 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many moles of bromide ions are present in 750.0 mL of 1.35 M MgBr2?
A)0.506 mol
B)1.01 mol
C)2.03 mol
D)3.04 mol
E)None of these choices is correct.
A)0.506 mol
B)1.01 mol
C)2.03 mol
D)3.04 mol
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
38
Procaine hydrochloride ( 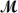
= 272.77 g/mol)is used as a local anesthetic.Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
A)2.272 M
B)4.056 M
C)4.216 M
D)4.666 M
E)None of these choices is correct.
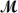
= 272.77 g/mol)is used as a local anesthetic.Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
A)2.272 M
B)4.056 M
C)4.216 M
D)4.666 M
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
39
Aqueous ammonia is commercially available in a solution that is 28% (w/w)ammonia.What is the mole fraction of ammonia in such a solution?
A)0.017
B)0.023
C)0.012
D)0.24
E)0.29
A)0.017
B)0.023
C)0.012
D)0.24
E)0.29

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
40
Children under the age of six with more than 0.10 ppm of lead in their blood can suffer a reduction in I.Q.or have behavior problems.What is the molality of a solution which contains 0.10 ppm of lead?
A)4.8 10-10 m
B)4.8 10-7 m
C)4.8 10-4 m
D)4.8 10-1 m
E)None of these choices is correct.
A)4.8 10-10 m
B)4.8 10-7 m
C)4.8 10-4 m
D)4.8 10-1 m
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
41
Cinnamaldehyde ( 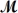
= 132.15 g/mol)is used as a flavoring agent.What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°C?
Kb = 1.22°C/m,boiling point of pure ethanol = 78.5°C
A)62.4 g
B)67.8 g
C)76.2 g
D)78.5 g
E)79.6 g
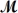
= 132.15 g/mol)is used as a flavoring agent.What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°C?
Kb = 1.22°C/m,boiling point of pure ethanol = 78.5°C
A)62.4 g
B)67.8 g
C)76.2 g
D)78.5 g
E)79.6 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
42
Lysine is an amino acid that is an essential part of nutrition but which is not synthesized by the human body.What is the molar mass of lysine if 750.0 mL of a solution containing 8.60 g of lysine has an osmotic pressure of 1.918 atm?
T = 25.0°C
A)110.g/mol
B)146 g/mol
C)220.g/mol
D)1340 g/mol
E)1780 g/mol
T = 25.0°C
A)110.g/mol
B)146 g/mol
C)220.g/mol
D)1340 g/mol
E)1780 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
43
Human blood has a molar concentration of solutes of 0.30 M.What is the osmotic pressure of blood at 25°C?
A)0.012 atm
B)0.62 atm
C)6.8 atm
D)7.3 atm
E)>10.atm
A)0.012 atm
B)0.62 atm
C)6.8 atm
D)7.3 atm
E)>10.atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
44
Safrole is used as a topical antiseptic.Calculate the vapor pressure of a solution prepared by dissolving 0.75 mol of safrole in 950 g of ethanol ( 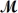
= 46.07 g/mol).P°ethanol = 50.0 torr at 25°C.
A)1.8 torr
B)11 torr
C)15 torr
D)40 torr
E)48 torr
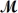
= 46.07 g/mol).P°ethanol = 50.0 torr at 25°C.
A)1.8 torr
B)11 torr
C)15 torr
D)40 torr
E)48 torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
45
Determine the freezing point of a solution which contains 0.31 mol of sucrose in 175 g of water.Kf = 1.86°C/m
A)3.3°C
B)1.1°C
C)0.0°C
D)-1.1°C
E)-3.3°C
A)3.3°C
B)1.1°C
C)0.0°C
D)-1.1°C
E)-3.3°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
46
Two aqueous solutions are prepared: 2.0 m Cu(NO3)2 and 2.0 m NaBr.Which of the following statements is true?
A)The Cu(NO3)2 solution has a higher vapor pressure and lower freezing point than the NaBr solution.
B)The Cu(NO3)2 solution has a higher vapor pressure and higher freezing point than the NaBr solution.
C)The Cu(NO3)2 solution has a lower vapor pressure and lower freezing point than the NaBr solution.
D)The Cu(NO3)2 solution has a lower vapor pressure and higher freezing point than the NaBr solution.
E)None of these statements is true.
A)The Cu(NO3)2 solution has a higher vapor pressure and lower freezing point than the NaBr solution.
B)The Cu(NO3)2 solution has a higher vapor pressure and higher freezing point than the NaBr solution.
C)The Cu(NO3)2 solution has a lower vapor pressure and lower freezing point than the NaBr solution.
D)The Cu(NO3)2 solution has a lower vapor pressure and higher freezing point than the NaBr solution.
E)None of these statements is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
47
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 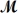
= 74.55 g/mol)in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
A)-1.7°C
B)-0.9°C
C)0.0°C
D)0.9°C
E)1.7°C
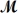
= 74.55 g/mol)in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
A)-1.7°C
B)-0.9°C
C)0.0°C
D)0.9°C
E)1.7°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following aqueous solutions will have the lowest osmotic pressure?
A)0.10 m KOH
B)0.10 m RbCl
C)0.05 m CaSO4
D)0.05 m BaCl2
E)0.10 m K2SO4
A)0.10 m KOH
B)0.10 m RbCl
C)0.05 m CaSO4
D)0.05 m BaCl2
E)0.10 m K2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
49
Hexachlorophene is used as a disinfectant in germicidal soaps.What mass of hexachlorophene ( 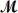
= 406.9 g/mol)must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C?
Kb = 3.63°C/m,boiling point of pure chloroform = 61.70°C
A)12.6 g
B)17.2 g
C)31.0 g
D)34.4 g
E)101 g
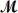
= 406.9 g/mol)must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C?
Kb = 3.63°C/m,boiling point of pure chloroform = 61.70°C
A)12.6 g
B)17.2 g
C)31.0 g
D)34.4 g
E)101 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
50
Dimethylglyoxime,DMG,is an organic compound used to test for aqueous nickel(II)ions.A solution prepared by dissolving 65.0 g of DMG in 375 g of ethanol boils at 80.3°C.What is the molar mass of DMG?
Kb = 1.22°C/m,boiling point of pure ethanol = 78.5°C
A)44.1 g/mol
B)65.8 g/mol
C)117 g/mol
D)131.6 g/mol
E)553 g/mol
Kb = 1.22°C/m,boiling point of pure ethanol = 78.5°C
A)44.1 g/mol
B)65.8 g/mol
C)117 g/mol
D)131.6 g/mol
E)553 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
51
Diethyl ether has a vapor pressure of 400.0 torr at 18°C.When a sample of benzoic acid is dissolved in ether,the vapor pressure of the solution is 342 torr.What is the mole fraction of benzoic acid in the solution?
A)0.0169
B)0.0197
C)0.145
D)0.855
E)None of these choices is correct.
A)0.0169
B)0.0197
C)0.145
D)0.855
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
52
Barbiturates are synthetic drugs used as sedatives and hypnotics.Barbital ( 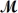
= 184.2 g/mol)is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid?
Kb = 3.07°C/m,boiling point of pure acetic acid = 117.9°C
A)117.0°C
B)117.7°C
C)118.1°C
D)118.8°C
E)>120°C
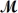
= 184.2 g/mol)is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid?
Kb = 3.07°C/m,boiling point of pure acetic acid = 117.9°C
A)117.0°C
B)117.7°C
C)118.1°C
D)118.8°C
E)>120°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following aqueous liquids will have the lowest freezing point?
A)0.5 m C12H22O11 (sucrose)
B)0.5 m Ca(NO3)2
C)0.5 m NiSO4
D)0.5 m Li3PO4
E)pure water
A)0.5 m C12H22O11 (sucrose)
B)0.5 m Ca(NO3)2
C)0.5 m NiSO4
D)0.5 m Li3PO4
E)pure water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
54
The density of pure water at 25°C is 0.997 g/mL.Considering water as being both solvent and solute,calculate its molarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
55
A 50.0 % (w/w)solution of sulfuric acid (H2SO4)in water has a density of 1.395 g/mL.What is its molarity?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
56
A 0.100 m K2SO4 solution has a freezing point of -0.43°C.What is the van't Hoff factor for this solution?
Kf = 1.86°C/m
A)0.77
B)1.0
C)2.3
D)3.0
E)>3.0
Kf = 1.86°C/m
A)0.77
B)1.0
C)2.3
D)3.0
E)>3.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
57
Benzaldehyde ( 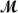
= 106.1 g/mol),also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m,freezing point of pure ethanol = -117.3°C.
A)-117.5°C
B)-118.7°C
C)-119.0°C
D)-120.6°C
E)< -121°C
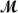
= 106.1 g/mol),also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m,freezing point of pure ethanol = -117.3°C.
A)-117.5°C
B)-118.7°C
C)-119.0°C
D)-120.6°C
E)< -121°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
58
Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 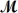
= 86.18 g/mol)at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
A)54 torr
B)154 torr
C)246 torr
D)346 torr
E)400.torr
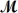
= 86.18 g/mol)at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
A)54 torr
B)154 torr
C)246 torr
D)346 torr
E)400.torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
59
A 0.100 m MgSO4 solution has a freezing point of -0.23°C.What is the van't Hoff factor for this solution?
Kf = 1.86°C/m
A)0.62
B)1.0
C)1.2
D)2.0
E)4.0
Kf = 1.86°C/m
A)0.62
B)1.0
C)1.2
D)2.0
E)4.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
60
Carbon tetrachloride,once widely used in fire extinguishers and as a dry cleaning fluid,has been found to cause liver damage to those exposed to its vapors over long periods of time.What is the boiling point of a solution prepared by dissolving 375 g of sulfur (S8, 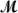
= 256.5g/mol)in 1250 g of CCl4? Kb = 5.05°C/m,boiling point of pure CCl4 = 76.7°C?
A)70.8°C
B)75.2°C
C)78.2°C
D)82.6°C
E)>85°C
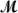
= 256.5g/mol)in 1250 g of CCl4? Kb = 5.05°C/m,boiling point of pure CCl4 = 76.7°C?
A)70.8°C
B)75.2°C
C)78.2°C
D)82.6°C
E)>85°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
61
If the density of a solution is less than 1.0 g/mL,its molarity will be greater than its molality.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
62
Octane and nonane are liquids which are components of gasoline.Their vapor pressures at 25°C are 13.9 torr and 4.7 torr,respectively.What is the vapor pressure of a mixture consisting of 1 mole of each of these compounds?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
63
A 7.112 M solution of sulfuric acid (H2SO4)in water has a density of 1.395 g/mL.What is its molality?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
64
If the shell of a raw egg is carefully dissolved away,and the egg in its flexible membrane is then placed in distilled water,the egg's volume will expand.Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
65
The solubility of gases in water increases with increase in the pressure of the gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
66
Predict the van't Hoff factor (i)for each of the following solutes:
a.CaCl2
b.Fe2(SO4)3
c.NH4Cl
a.CaCl2
b.Fe2(SO4)3
c.NH4Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
67
Heats of solution may be either positive or negative.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
68
Heats of hydration may be either positive or negative.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
69
In general,water is a good solvent for both polar and non-polar compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
70
A 0.137 m solution of sodium ferrocyanide (NaFe(CN)6.10H2O)in water has a freezing point depression of 0.755°C.Calculate the van't Hoff factor (i)under these conditions.(Kf = 1.86°C/m)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
71
Raoult's Law relates the vapor pressure of a solvent above a solution to the mole fraction of the solvent and the vapor pressure of the pure solvent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
72
The heat of solution is the total enthalpy change when a solution is formed from the separated solute and solvent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
73
Ideal solutions do not conform to Raoult's Law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck








