Deck 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/84
العب
ملء الشاشة (f)
Deck 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions
1
A certain process has Suniv > 0 at 25°C.What does one know about the process?
A)It is exothermic.
B)It is endothermic.
C)It is spontaneous at 25°C.
D)It will move rapidly toward equilibrium.
E)None of these choices is correct.
A)It is exothermic.
B)It is endothermic.
C)It is spontaneous at 25°C.
D)It will move rapidly toward equilibrium.
E)None of these choices is correct.
It is spontaneous at 25°C.
2
Which relationship or statement best describes S° for the following reaction?
C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
( S° < 0)
3
Which of the following results in a decrease in the entropy of the system?
A)O2(g),300 K O2(g),400 K
B)H2O(s),0°C H2O(l),0°C
C)N2(g),25°C N2(aq),25°C
D)NH3(l),-34.5°C NH3(g),-34.5°C
E)2H2O2(g) 2H2O(g)+ O2(g)
A)O2(g),300 K O2(g),400 K
B)H2O(s),0°C H2O(l),0°C
C)N2(g),25°C N2(aq),25°C
D)NH3(l),-34.5°C NH3(g),-34.5°C
E)2H2O2(g) 2H2O(g)+ O2(g)
N2(g),25°C N2(aq),25°C
4
Which of the following is always true for an endothermic process?
A)q sys > 0, Ssurr < 0
B)qsys < 0, Ssurr > 0
C)qsys < 0, Ssurr < 0
D)qsys > 0, Ssurr > 0
E)w < 0
A)q sys > 0, Ssurr < 0
B)qsys < 0, Ssurr > 0
C)qsys < 0, Ssurr < 0
D)qsys > 0, Ssurr > 0
E)w < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which relationship or statement best describes S° for the following reaction?
Pb(s)+ Cl2(g) PbCl2(s)
A)( S° 00
B)9 S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
Pb(s)+ Cl2(g) PbCl2(s)
A)( S° 00
B)9 S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which,if any,of the following processes is spontaneous under the specified conditions?
A)H2O(l) H2O(s)at 25°C
B)CO2(s) CO2(g)at 0°C
C)2H2O(g) 2H2(g)+ O2(g)
D)C(graphite) C(diamond)at 25°C and 1 atm pressure
E)None of these is spontaneous.
A)H2O(l) H2O(s)at 25°C
B)CO2(s) CO2(g)at 0°C
C)2H2O(g) 2H2(g)+ O2(g)
D)C(graphite) C(diamond)at 25°C and 1 atm pressure
E)None of these is spontaneous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following values is based on the Third Law of Thermodynamics?
A)( H° f = 0 for Al(s)at 298 K)
B)( G° f = 0 for H2(g)at 298 K)
C)S° = 51.446 J/(mol·K)for Na(s)at 298 K
D)q sys < 0 for H2O(l) H2O(s)at 0°C
E)None of these choices is correct.
A)( H° f = 0 for Al(s)at 298 K)
B)( G° f = 0 for H2(g)at 298 K)
C)S° = 51.446 J/(mol·K)for Na(s)at 298 K
D)q sys < 0 for H2O(l) H2O(s)at 0°C
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which relationship or statement best describes S° for the following reaction?
2H2S(g)+ 3O2(g) 2H2O(g)+ 2SO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
2H2S(g)+ 3O2(g) 2H2O(g)+ 2SO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
9
When a sky diver free-falls through the air,the process is
A)non-spontaneous because he is accelerating due to the force applied by gravity.
B)non-spontaneous because he is losing potential energy.
C)non-spontaneous because he had planned the jump for two weeks.
D)spontaneous.
E)in equilibrium.
A)non-spontaneous because he is accelerating due to the force applied by gravity.
B)non-spontaneous because he is losing potential energy.
C)non-spontaneous because he had planned the jump for two weeks.
D)spontaneous.
E)in equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following is always true for an exothermic process?
A)qsys > 0, Ssurr < 0
B)qsys < 0, Ssurr > 0
C)qsys < 0, Ssurr < 0
D)qsys > 0, Ssurr > 0
E)w < 0
A)qsys > 0, Ssurr < 0
B)qsys < 0, Ssurr > 0
C)qsys < 0, Ssurr < 0
D)qsys > 0, Ssurr > 0
E)w < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which relationship or statement best describes S° for the following reaction?
O3(g)+ NO(g) O2(g)+ NO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
O3(g)+ NO(g) O2(g)+ NO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following is true for a system at equilibrium?
A)( S°sys = S°surr)
B)( S°sys = - S°surr)
C)( S°sys = S°surr = 0)
D)( S°univ > 0)
E)(None of these is a sufficient condition.
A)( S°sys = S°surr)
B)( S°sys = - S°surr)
C)( S°sys = S°surr = 0)
D)( S°univ > 0)
E)(None of these is a sufficient condition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which relationship best describes S° for the following reaction?
8H2(g)+ S8(s) 8H2S(g)
A)9 S° = H°)
B)( S° = H°/T)
C)( S° )
D)( S° < 0)
E)( S° > 0)
8H2(g)+ S8(s) 8H2S(g)
A)9 S° = H°)
B)( S° = H°/T)
C)( S° )
D)( S° < 0)
E)( S° > 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following should have the greatest molar entropy at 298 K?
A)CH4(g)
B)H2O(l)
C)NaCl(s)
D)N2O4(g)
E)H2(g)
A)CH4(g)
B)H2O(l)
C)NaCl(s)
D)N2O4(g)
E)H2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which relationship or statement best describes S° for the following reaction?
2NH3(g)+ 2ClF3(g) 6HF(g)+ N2(g)+ Cl2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
2NH3(g)+ 2ClF3(g) 6HF(g)+ N2(g)+ Cl2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is necessary for a process to be spontaneous?
A)( Hsys < 0)
B)( Ssys > 0)
C)( Ssurr < 0)
D)( Suniv > 0)
E)( Gsys = 0)
A)( Hsys < 0)
B)( Ssys > 0)
C)( Ssurr < 0)
D)( Suniv > 0)
E)( Gsys = 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following is true for pure oxygen gas,O2(g)at 25°C?
A)( H° f > 0)
B)( H° f < 0)
C)( G° f > 0)
D)( G° f < 0)
E)(S° > 0)
A)( H° f > 0)
B)( H° f < 0)
C)( G° f > 0)
D)( G° f < 0)
E)(S° > 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which relationship or statement best describes S° for the following reaction?
K2SO4(s) 2K+(aq)+ SO42-(aq)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
K2SO4(s) 2K+(aq)+ SO42-(aq)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which relationship best describes S° for the following reaction?
CO(g)+ H2O(g) CO2(g)+ H2(g)
A)( S° = H°)
B)(F S° = H°/T)
C)( S° > 0)
D)( S° < 0)
E)( S° 0)
CO(g)+ H2O(g) CO2(g)+ H2(g)
A)( S° = H°)
B)(F S° = H°/T)
C)( S° > 0)
D)( S° < 0)
E)( S° 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which relationship or statement best describes S° for the following reaction?
HgS(s)+ O2(g) Hg(l)+ SO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
HgS(s)+ O2(g) Hg(l)+ SO2(g)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
21
For a chemical reaction to be spontaneous at all temperatures,which of the following conditions must be met?
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)(It is not possible for a reaction to be spontaneous at all temperatures.
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)(It is not possible for a reaction to be spontaneous at all temperatures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
22
Elemental boron can be formed by reaction of boron trichloride with hydrogen.
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
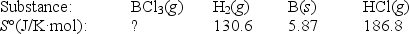
If S° = 80.3 J/K,what is S° for BCl3(g)?
A)-18.2 J/K·mol
B)18.2 J/K·mol
C)290.1 J/K·mol
D)355.4 J/K·mol
E)450.6 J/K·mol
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
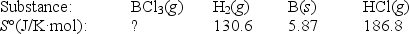
If S° = 80.3 J/K,what is S° for BCl3(g)?
A)-18.2 J/K·mol
B)18.2 J/K·mol
C)290.1 J/K·mol
D)355.4 J/K·mol
E)450.6 J/K·mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
23
Calculate S° for the reaction
2Cl2(g)+ SO2(g) SOCl2(g)+ Cl2O(g)
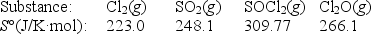
A)-118.2 J/K
B)-104.8 J/K
C)104.8 J/K
D)118.2 J/K
E)1270.0 J/K
2Cl2(g)+ SO2(g) SOCl2(g)+ Cl2O(g)
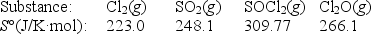
A)-118.2 J/K
B)-104.8 J/K
C)104.8 J/K
D)118.2 J/K
E)1270.0 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
24
Calculate S° for the combustion of propane.
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(g)
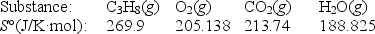
A)-100.9 J/K
B)-72.5 J/K
C)72.5 J/K
D)100.9 J/K
E)877.5 J/K
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(g)
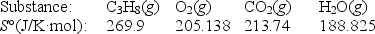
A)-100.9 J/K
B)-72.5 J/K
C)72.5 J/K
D)100.9 J/K
E)877.5 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
25
In which one of these pairs will the entropy of the first substance be greater than that of the second? Assume P and T are the same for each pair,unless stated otherwise.
A)1 mole of F2(g);1 mole of Cl2(g)
B)1 mole of I2(s);1 mole of I2(g)
C)1 mole of CaCO3(s);1 mole of CaO(s)plus 1 mole of CO2(g)
D)1 mole of H2(g)at 25°C;1 mole of H2(g)at 50°C
E)1 mole of O3(g);1 mole of O2(g)
A)1 mole of F2(g);1 mole of Cl2(g)
B)1 mole of I2(s);1 mole of I2(g)
C)1 mole of CaCO3(s);1 mole of CaO(s)plus 1 mole of CO2(g)
D)1 mole of H2(g)at 25°C;1 mole of H2(g)at 50°C
E)1 mole of O3(g);1 mole of O2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
26
Calculate S° for the reaction
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s)
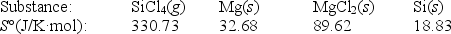
A)-254.96 J/K
B)-198.02 J/K
C)198.02 J/K
D)254.96 J/K
E)471.86 J/K
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s)
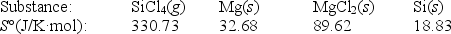
A)-254.96 J/K
B)-198.02 J/K
C)198.02 J/K
D)254.96 J/K
E)471.86 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
27
For a process with S < 0,which one of the following statements is correct?
A)The process will definitely be spontaneous if H < 0.
B)The process will be definitely be spontaneous if H < T S.
C)The process can never be spontaneous.
D)The process will definitely be spontaneous,regardless of H.
E)The process will definitely be spontaneous if Ssurr > 0.
A)The process will definitely be spontaneous if H < 0.
B)The process will be definitely be spontaneous if H < T S.
C)The process can never be spontaneous.
D)The process will definitely be spontaneous,regardless of H.
E)The process will definitely be spontaneous if Ssurr > 0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
28
You are given pure samples of ethane,C2H6(g),and toluene,C7H8(l).What prediction would you make concerning their standard molar entropies at 298 K?
A)S°ethane > S°toluene
B)S°ethane < S°toluene
C)S°ethane (S°toluene) 3
D)S°ethane S°toluene
E)Since toluene is much more complex than ethane,but ethane is in the gas phase while toluene is a liquid,none of these predictions can be confidently made without further information or calculations.
A)S°ethane > S°toluene
B)S°ethane < S°toluene
C)S°ethane (S°toluene) 3
D)S°ethane S°toluene
E)Since toluene is much more complex than ethane,but ethane is in the gas phase while toluene is a liquid,none of these predictions can be confidently made without further information or calculations.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider the following quantities used in thermodynamics: E,H,q,w,S,G.How many of them are state functions?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which relationship or statement best describes S° for the following reaction?
CaO(s)+ CO2(g) CaCO3(s)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
CaO(s)+ CO2(g) CaCO3(s)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
31
In which one of the following pairs will the first system have a higher entropy than the second? Assume P and T are the same for each pair,unless stated otherwise.
A)1 mole He(g);1 mole Kr(g)
B)1 mole O2(g);2 mole O(g)
C)1 mole CH4(g);1 mole C2H6(g)
D)1 mole Xe(g)at 1 atmosphere;1 mole Xe(g)at 0.5 atmosphere
E)20 one-dollar bills distributed randomly among 20 people;20 one-dollar bills distributed randomly among 10 people
A)1 mole He(g);1 mole Kr(g)
B)1 mole O2(g);2 mole O(g)
C)1 mole CH4(g);1 mole C2H6(g)
D)1 mole Xe(g)at 1 atmosphere;1 mole Xe(g)at 0.5 atmosphere
E)20 one-dollar bills distributed randomly among 20 people;20 one-dollar bills distributed randomly among 10 people

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
32
You are given pure samples of ammonia,NH3(g),and nitrogen trifluoride,NF3(g).What prediction would you make concerning their standard molar entropies at 298 K?
A)S°ammonia > S°nitrogen trifluoride
B)S°ammonia < S°nitrogen trifluoride
C)S°ammonia S°nitrogen trifluoride
D)Other conditions need to be specified before a reliable prediction can be made.
E)Even if more conditions are specified,a reliable prediction cannot be made.
A)S°ammonia > S°nitrogen trifluoride
B)S°ammonia < S°nitrogen trifluoride
C)S°ammonia S°nitrogen trifluoride
D)Other conditions need to be specified before a reliable prediction can be made.
E)Even if more conditions are specified,a reliable prediction cannot be made.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
33
For a chemical reaction to be spontaneous only at high temperatures,which of the following conditions must be met?
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)( G° > 0)
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)( G° > 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following pairs has the member with the greater molar entropy listed first? All systems are at 25°C.
A)CO(g),CO2(g)
B)NaCl(s),NaCl(aq)
C)H2S(g),H2S(aq)
D)Li(s),Pb(s)
E)H2(g),H2O(g)
A)CO(g),CO2(g)
B)NaCl(s),NaCl(aq)
C)H2S(g),H2S(aq)
D)Li(s),Pb(s)
E)H2(g),H2O(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
35
For a chemical reaction to be spontaneous only at low temperatures,which of the following conditions must be met?
A)( S°·> 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)( G° > 0)
A)( S°·> 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)( G° > 0)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
36
For a chemical reaction to be non-spontaneous at any temperature,which of the following conditions must be met?
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)(All reactions are spontaneous at some temperature.
A)( S° > 0, H° > 0)
B)( S° > 0, H° < 0)
C)( S° < 0, H° < 0)
D)( S° < 0, H° > 0)
E)(All reactions are spontaneous at some temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which relationship or statement best describes S° for the following reaction?
BaCl2(aq)+ Na2SO4(aq) BaSO4(s)+ 2NaCl(aq)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.
BaCl2(aq)+ Na2SO4(aq) BaSO4(s)+ 2NaCl(aq)
A)( S° 0)
B)( S° < 0)
C)( S° > 0)
D)( S° = H°/T)
E)More information is needed to make a reasonable prediction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
38
Calculate S° for the reaction
4Cr(s)+ 3O2(g) 2Cr2O3(s)
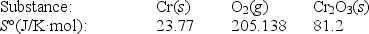
A)-548.1 J/K
B)-147.7 J/K
C)147.7 J/K
D)310.1 J/K
E)548.1 J/K
4Cr(s)+ 3O2(g) 2Cr2O3(s)
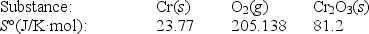
A)-548.1 J/K
B)-147.7 J/K
C)147.7 J/K
D)310.1 J/K
E)548.1 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
39
You are given pure samples of pentane,CH3CH2CH2CH2CH3(l),and 1,3-pentadiene,CH2=CHCH=CHCH3(l).What prediction would you make concerning the standard molar entropies of pentane,S°(pentane)and 1,3-pentadiene,S°(1,3-pentadiene),at 298 K?
A)S°(pentane)> S°(1,3-pentadiene)
B)S°(pentane)< S°(1,3-pentadiene)
C)S°(pentane) S°(1,3-pentadiene)
D)S°(pentane)= S°(1,3-pentadiene)+ 2 S°(H2)
E)More information is needed to make reasonable predictions.
A)S°(pentane)> S°(1,3-pentadiene)
B)S°(pentane)< S°(1,3-pentadiene)
C)S°(pentane) S°(1,3-pentadiene)
D)S°(pentane)= S°(1,3-pentadiene)+ 2 S°(H2)
E)More information is needed to make reasonable predictions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
40
In order for a process to be spontaneous,
A)( H must be less than zero.)
B)( S must be greater than zero.)
C)( G must be greater than zero.)
D)it should be rapid.
E)( Ssys + Ssurr must be greater than zero.)
A)( H must be less than zero.)
B)( S must be greater than zero.)
C)( G must be greater than zero.)
D)it should be rapid.
E)( Ssys + Ssurr must be greater than zero.)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
41
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.From this plot,it is reasonable to conclude that: 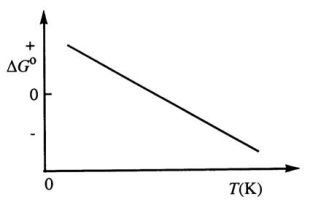
A)( H° > 0, S° > 0)
B)( H° > 0, S° < 0)
C)9 H° < 0, S° > 0)
D)( H° < 0, S° < 0)
E)None of these choices is correct.

A)( H° > 0, S° > 0)
B)( H° > 0, S° < 0)
C)9 H° < 0, S° > 0)
D)( H° < 0, S° < 0)
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
42
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine.The data refer to 298 K.
SO2(g)+ Cl2(g) SO2Cl2(g)
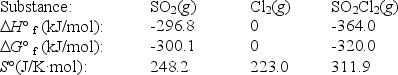
What is the value of G° for this reaction at 600 K?
A)-162.8 kJ
B)-40.1 kJ
C)-28.4 kJ
D)28.4 kJ
E)162.8 kJ
SO2(g)+ Cl2(g) SO2Cl2(g)
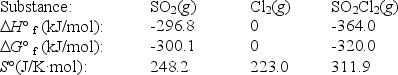
What is the value of G° for this reaction at 600 K?
A)-162.8 kJ
B)-40.1 kJ
C)-28.4 kJ
D)28.4 kJ
E)162.8 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
43
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.Which one of the following is an incorrect conclusion,based on the information in the diagram? 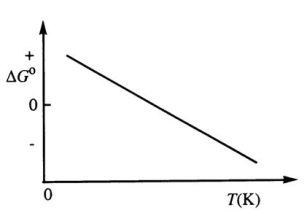
A)( H° > 0)
B)( S° > 0)
C)The reaction is spontaneous at high temperatures.
D)( S° increases with temperature while H° remains constant.)
E)(There exists a certain temperature at which H° = T S°.)

A)( H° > 0)
B)( S° > 0)
C)The reaction is spontaneous at high temperatures.
D)( S° increases with temperature while H° remains constant.)
E)(There exists a certain temperature at which H° = T S°.)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
44
Nitric oxide reacts with chlorine to form NOCl.The data refer to 298 K.
2NO(g)+ Cl2(g) 2NOCl(g)
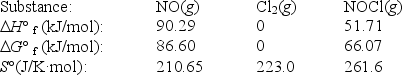
What is the value of G° for this reaction at 550 K?
A)-143.76 kJ
B)-78.78 kJ
C)-22.24 kJ
D)-10.56 kJ
E)66,600 kJ
2NO(g)+ Cl2(g) 2NOCl(g)
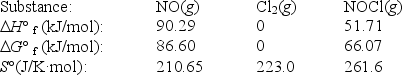
What is the value of G° for this reaction at 550 K?
A)-143.76 kJ
B)-78.78 kJ
C)-22.24 kJ
D)-10.56 kJ
E)66,600 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
45
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen.The data refer to 25°C.
CH4(g)+ 2H2O(g)
CO2(g)+ 4H2(g)

A)8.2 1019
B)0.96
C)0.58
D)1.2 10-20
E)1.4 10-46
CH4(g)+ 2H2O(g)

CO2(g)+ 4H2(g)

A)8.2 1019
B)0.96
C)0.58
D)1.2 10-20
E)1.4 10-46

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the free energy change, G°,for the equilibrium between hydrogen iodide,hydrogen,and iodine at 453°C? Kc = 0.020
2HI(g)
H2(g)+ I2(g)
A)6.4 kJ
B)8.8 kJ
C)15 kJ
D)19 kJ
E)24 kJ
2HI(g)

H2(g)+ I2(g)
A)6.4 kJ
B)8.8 kJ
C)15 kJ
D)19 kJ
E)24 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
47
The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K.At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K.
CH4(g)+ 2H2O(g)
CO2(g)+ 4H2(g)

A)658 K
B)683 K
C)955 K
D)1047 K
E)1229 K
CH4(g)+ 2H2O(g)

CO2(g)+ 4H2(g)

A)658 K
B)683 K
C)955 K
D)1047 K
E)1229 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
48
Iron(III)oxide can be reduced by carbon monoxide.
Fe2O3(s)+ 3CO(g)
2Fe(s)+ 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
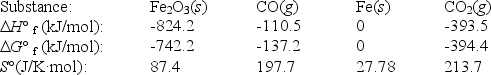
A)7.0 10-6
B)1.3 10-3
C)2.2 104
D)1.4 105
E) > 2.0 105
Fe2O3(s)+ 3CO(g)

2Fe(s)+ 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
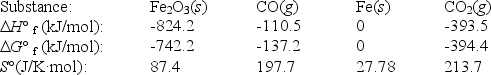
A)7.0 10-6
B)1.3 10-3
C)2.2 104
D)1.4 105
E) > 2.0 105

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
49
a.Explain what is meant by a spontaneous process.
b.Is a spontaneous process necessarily a rapid one? Explain,and provide a real reaction as an example to illustrate your answer.
b.Is a spontaneous process necessarily a rapid one? Explain,and provide a real reaction as an example to illustrate your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
50
Use the given data at 298 K to calculate G° for the reaction
2Cl2(g)+ SO2(g) SOCl2(g)+ Cl2O(g)
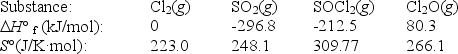
A)129.3 kJ
B)133.6 kJ
C)196.0 kJ
D)199.8 kJ
E)229.6 kJ
2Cl2(g)+ SO2(g) SOCl2(g)+ Cl2O(g)
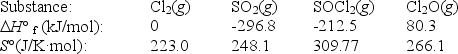
A)129.3 kJ
B)133.6 kJ
C)196.0 kJ
D)199.8 kJ
E)229.6 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
51
Calculate G° for the reaction of ammonia with fluorine.
2NH3(g)+ 5F2(g) N2F4(g)+ 6HF(g)
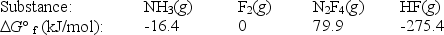
A)179.1 kJ
B)-179.1 kJ
C)1539.7 kJ
D)-1539.7 kJ
E)None of these choices is correct.
2NH3(g)+ 5F2(g) N2F4(g)+ 6HF(g)
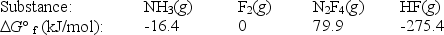
A)179.1 kJ
B)-179.1 kJ
C)1539.7 kJ
D)-1539.7 kJ
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
52
Use the thermodynamic data at 298 K below to determine the Ksp for barium carbonate,BaCO3 at this temperature.
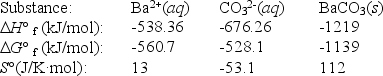
A)5.86
B)6.30 108
C)1.59 10-9
D)5.47 10-21
E)2.18 10-27
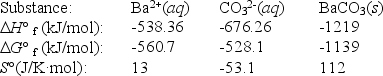
A)5.86
B)6.30 108
C)1.59 10-9
D)5.47 10-21
E)2.18 10-27

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
53
Calculate G° for the reaction
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s)

A)566.60 kJ
B)50.38 kJ
C)25.19 kJ
D)-25.19 kJ
E)-566.60 kJ
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s)

A)566.60 kJ
B)50.38 kJ
C)25.19 kJ
D)-25.19 kJ
E)-566.60 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
54
Hydrogen sulfide decomposes according to the following reaction
2H2S(g) 2H2(g)+ S2(g)
For this reaction at 298K S° = 78.1 J/K, H° = 169.4 kJ,and G° = 146.1 kJ.What is the value of G° at 900 K?
A)-69,881 kJ
B)48.4 kJ
C)99.1 kJ
D)240 kJ
E)441 kJ
2H2S(g) 2H2(g)+ S2(g)
For this reaction at 298K S° = 78.1 J/K, H° = 169.4 kJ,and G° = 146.1 kJ.What is the value of G° at 900 K?
A)-69,881 kJ
B)48.4 kJ
C)99.1 kJ
D)240 kJ
E)441 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
55
Calculate G° for the combustion of propane.
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(g)

A)-2073.1 kJ
B)-1387.3 kJ
C)-598.5 kJ
D)598.5 kJ
E)2073.1 kJ
C3H8(g)+ 5O2(g) 3CO2(g)+ 4H2O(g)

A)-2073.1 kJ
B)-1387.3 kJ
C)-598.5 kJ
D)598.5 kJ
E)2073.1 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
56
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid.
H3PO4(s)
H3PO4(l)
Use the following thermodynamic data at 298 K to determine this temperature.

A)286 K
B)305 K
C)315 K
D)347 K
E)3170 K
H3PO4(s)

H3PO4(l)
Use the following thermodynamic data at 298 K to determine this temperature.

A)286 K
B)305 K
C)315 K
D)347 K
E)3170 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
57
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide.
H2O2(l)
H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.
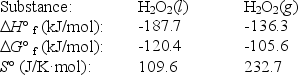
A)120°C
B)144°C
C)196°C
D)418°C
E)585°C
H2O2(l)

H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.
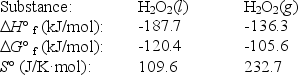
A)120°C
B)144°C
C)196°C
D)418°C
E)585°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
58
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.From this plot,it is reasonable to conclude that: 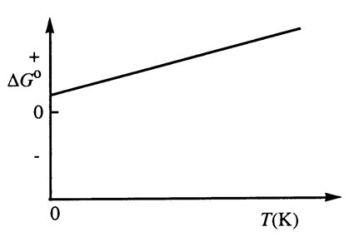
A)( H° > 0, S° > 0)
B)( H° > 0, S° < 0)
C)( H° < 0, S° > 0)
D)( H° < 0, S° < 0)
E)None of these choices is correct.

A)( H° > 0, S° > 0)
B)( H° > 0, S° < 0)
C)( H° < 0, S° > 0)
D)( H° < 0, S° < 0)
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
59
The formation constant for the reaction
Ag+(aq)+ 2NH3(aq)
Ag(NH3)2+(aq)
Is Kf = 1.7 107 at 25°C.What is G° at this temperature?
A)-1.5 kJ
B)-3.5 kJ
C)-18 kJ
D)-23 kJ
E)-41 kJ
Ag+(aq)+ 2NH3(aq)

Ag(NH3)2+(aq)
Is Kf = 1.7 107 at 25°C.What is G° at this temperature?
A)-1.5 kJ
B)-3.5 kJ
C)-18 kJ
D)-23 kJ
E)-41 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
60
Elemental boron can be formed by reaction of boron trichloride with hydrogen.
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
Calculate G° for the reaction.
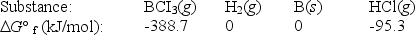
A)-293.4 kJ
B)293.4 kJ
C)-102.8 kJ
D)102.8 kJ
E)None of these choices is correct.
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
Calculate G° for the reaction.
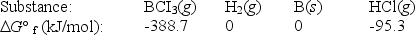
A)-293.4 kJ
B)293.4 kJ
C)-102.8 kJ
D)102.8 kJ
E)None of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
61
The higher the pressure of a gas sample,the greater is its entropy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
62
Given: C2H2(g) 2C(graphite)+ H2(g) G° = -209 kJ
A sample of gaseous C2H2 (acetylene,or ethyne)was stored for one year,yet at the end of this period the sample remained unchanged and no graphite or hydrogen gas had been formed.Briefly explain why there is no inconsistency between the sign of G° and the apparent stability of the sample.
A sample of gaseous C2H2 (acetylene,or ethyne)was stored for one year,yet at the end of this period the sample remained unchanged and no graphite or hydrogen gas had been formed.Briefly explain why there is no inconsistency between the sign of G° and the apparent stability of the sample.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
63
In some spontaneous processes,the entropy of the surroundings decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
64
The term microstate refers to the energy state of a single molecule in a system of many molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
65
The water-gas shift reaction plays an important role in the production of clean fuel from coal.
CO(g)+ H2O(g)
CO2(g)+ H2(g)
Use the following thermodynamic data to determine the equilibrium constant Kp at 700.K.
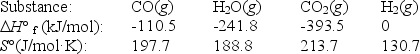
CO(g)+ H2O(g)

CO2(g)+ H2(g)
Use the following thermodynamic data to determine the equilibrium constant Kp at 700.K.
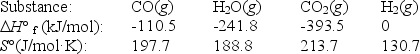

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
66
For the reaction of xenon and fluorine gases to form solid XeF4, H° = -251 kJ and G° = -121 kJ at 25°C.Calculate S° for the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
67
Compare one mole of ice with one mole of liquid water,both at 1.0 atm and 0°C.The melting point of ice at 1.0 atm is 0°C.For the process
H2O(s) H2O(l)
under these conditions predict whether each of the following quantities will be greater than,less than,or equal to,zero .Explain each prediction in one sentence.
a. H°
b. S°
c. G°
H2O(s) H2O(l)
under these conditions predict whether each of the following quantities will be greater than,less than,or equal to,zero .Explain each prediction in one sentence.
a. H°
b. S°
c. G°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
68
For what signs of H and S will a process
a.be spontaneous at high temperatures but not at low temperatures?
b.not be spontaneous at any temperatures?
a.be spontaneous at high temperatures but not at low temperatures?
b.not be spontaneous at any temperatures?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
69
In a spontaneous process,the entropy of the system always increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
70
For each of the following pairs,predict which (A or B)will have the greater entropy,and in one sentence indicate your reasoning.
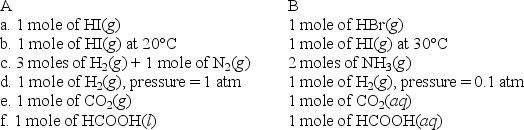
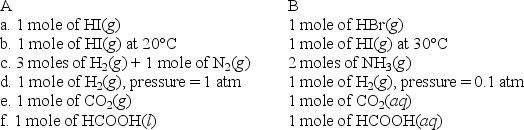

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
71
State the second and third laws of thermodynamics.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
72
In tables of thermodynamic data provided in chemistry books,one finds H° f , G° f and S° listed.Briefly,explain why the entropy data are supplied as S°,while the enthalpy and free energy data are in the form of H° f and G° f,respectively.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
73
In the expression,S = k ln W,W is called the number of microstates.Explain clearly the meaning of the word "microstate",and why a system under a given set of conditions normally has many microstates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
74
A reaction has a positive value of H° and a positive value of S°.
Draw a neat,labeled schematic plot to show how G° (y-axis)will depend on absolute temperature (x-axis).
Draw a neat,labeled schematic plot to show how G° (y-axis)will depend on absolute temperature (x-axis).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
75
A chemical reaction has G° = 10.0 kJ and S° = 50.0 J/K
a.Calculate H° for this reaction at 25°C.
b.Could this reaction ever be spontaneous? Explain your answer.
a.Calculate H° for this reaction at 25°C.
b.Could this reaction ever be spontaneous? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
76
For a reaction at equilibrium, Suniv = 0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
77
A chemical reaction has H° = 42.8 kJ and S° = 92.5 J/K,at 25°C.Calculate the temperature at which G° = 0.State any approximation involved in your calculation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
78
The complete combustion of liquid benzene is represented by the equation:
C6H6(l)+ 7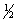
O2(g) 6CO2(g)+ 3H2O(l)
Using the data below,calculate,for this reaction
a. H°
b. S°
c. G° at 25°C.
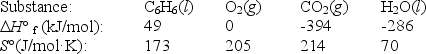
C6H6(l)+ 7
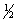
O2(g) 6CO2(g)+ 3H2O(l)
Using the data below,calculate,for this reaction
a. H°
b. S°
c. G° at 25°C.
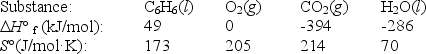

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
79
Photosynthesis can be represented by the equation
6CO2(g)+ 6H2O(l) C6H12O6(s)+ 6O2(g)
a.Calculate S° for this process,given the following data:
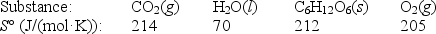
b.Given that H° for the reaction is 2802 kJ,calculate G° at 25°C.
6CO2(g)+ 6H2O(l) C6H12O6(s)+ 6O2(g)
a.Calculate S° for this process,given the following data:
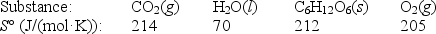
b.Given that H° for the reaction is 2802 kJ,calculate G° at 25°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck
80
Under a given set of conditions,all microstates of a system are equally probable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 84 في هذه المجموعة.
فتح الحزمة
k this deck








