Deck 11: Gases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/112
العب
ملء الشاشة (f)
Deck 11: Gases
1
For all gas law calculations,the temperature must be in kelvins.
True
2
Pressure depends on how many gas particles are in a container.
True
3
Gases are a collection of particles in constant,unpredictable motion.
False
4
As you increase temperature,you increase the average energy of the gas particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
5
Gas particles lose energy every time they collide with each other or the container wall.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
6
Straws work because sucking creates a pressure difference.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
7
As we climb a mountain to a higher altitude,we experience a pressure decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
8
The conversion factor for pressure is 1 mm Hg = 1 atm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
9
Gases fill the entire volume of their container.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
10
Pressure is calculated by: P = 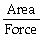
.
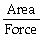
.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
11
Gases and liquids are compressible,but solids are not.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
12
The unit of pressure known as the atmosphere (atm)is defined as the average pressure found at the top of Mount Everest.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
13
Boyle's law states that as the volume of a gas increases so does the pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
14
If the column of mercury in a barometer drops to a lower reading,this means the measured pressure has decreased.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
15
The expected order of density for matter is gases < liquids < solids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
16
There is a large distance between gas particles as compared to their relative size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
17
Gas particles act independently of each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
18
The volume of a gas is independent of the temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
19
A sealed bag of potato chips will expand when taken to a higher altitude.This is an example of Boyle's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
20
A pedometer is a device created by Torricelli to measure pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
21
"Molar Mass" can be calculated using the formula: Molar mass = 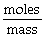
.
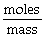
.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
22
Absolute zero refers to 0°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
23
If the temperature of a gas is doubled,the volume is doubled provided that the pressure and the number of particles remains constant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following statements about pressure is FALSE?
A)Pressure is caused by gas molecules colliding with surfaces.
B)The atmosphere has a pressure as the components of air collide with surfaces.
C)After creating a pressure difference,the atmospheric pressure can push liquid up a straw.
D)A deep well dug in the ground must have the pump located at the bottom of well in order to have the water come to the surface.
E)All of the above statements are true.
A)Pressure is caused by gas molecules colliding with surfaces.
B)The atmosphere has a pressure as the components of air collide with surfaces.
C)After creating a pressure difference,the atmospheric pressure can push liquid up a straw.
D)A deep well dug in the ground must have the pump located at the bottom of well in order to have the water come to the surface.
E)All of the above statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
25
One mole of F2 gas at STP would take up twice the volume of one mole of Ar gas at STP.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
26
If a slow leak in an inner tube has reduced the volume of the tube to one-third its original inflated volume,this means that one-third of the moles of gas have escaped the tube.(Assume constant temperature and pressure. )

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
27
The molar volume of any gas at conditions of standard temperature and pressure is 22.4 liters.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
28
If the number of gas particles is halved,the volume of the gas will be halved given that the temperature and pressure do not change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
29
The volume of 9.00 grams of water vapor at STP is 11.2 L.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
30
Using pure oxygen in scuba diving tanks is a good method of preventing the nitrogen bends.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
31
Charles's Law provides an explanation of why hot air balloons float.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
32
Vapor pressure of water increases with increasing temperature because the higher temperature causes more water molecules to evaporate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
33
Dalton's law of partial pressure is: P = 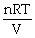
.
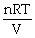
.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
34
A gas may not behave ideally under conditions of low pressure or high temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
35
STP conditions are 273 K and 760 mm Hg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
36
The volume of a gas and the number of particles is inversely proportional.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
37
If the number of gas particles is tripled,the volume will be 1/3 of the original given that temperature and pressure do not change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
38
The main component of the air we breathe is oxygen gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
39
The vapor pressure of water is independent of temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
40
If you had a five liter balloon of argon gas and a five liter balloon of xenon gas,and you removed 10 grams of gas from each balloon,the balloons would both shrink down to the same size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the equivalent pressure of 760 torr in units of mm Hg?
A)760
B)1
C)14.7
D)29.92
E)none of the above
A)760
B)1
C)14.7
D)29.92
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
42
To solve problems using Charles's Law,which mathematical equation should be used?
A)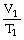
=
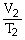
B)T1V1 = T2V2
C)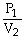
=
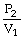
D)P2V1 = P1V2
E)none of the above
A)
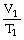
=
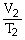
B)T1V1 = T2V2
C)
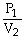
=
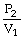
D)P2V1 = P1V2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
43
The initial volume of a gas cylinder is 750.0 mL.If the pressure of a gas inside the cylinder changes from
840.0 mm Hg to 360.0 mm Hg,what is the final volume the gas occupies?
A)3.151 L
B)630.0 mL
C)1.750 L
D)321.4 mL
E)none of the above
840.0 mm Hg to 360.0 mm Hg,what is the final volume the gas occupies?
A)3.151 L
B)630.0 mL
C)1.750 L
D)321.4 mL
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
44
To solve problems using Boyle's Law,which mathematical equation should be used?
A)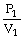
=
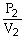
B)P1V1 = P2V2
C)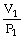
=
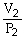
D)P2V1 = P1V2
E)none of the above
A)
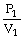
=
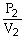
B)P1V1 = P2V2
C)
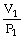
=
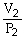
D)P2V1 = P1V2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
45
Divers often inflate heavy duty balloons attached to salvage items on the sea floor.If a balloon is filled to a volume of 3.00 L at a pressure of 2.50 atm,what is the volume of the balloon when it reaches the surface?
A)7.50 L
B)1.20 L
C)0.833 L
D)5.50 L
E)none of the above
A)7.50 L
B)1.20 L
C)0.833 L
D)5.50 L
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
46
1 torr is equal to:
A)760 mm Hg.
B)1 mm Hg.
C)1 Pa.
D)14.7 psi.
E)all of the above
A)760 mm Hg.
B)1 mm Hg.
C)1 Pa.
D)14.7 psi.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
47
1 atm is equal to:
A)760 mm Hg.
B)760 torr.
C)101,325 Pa.
D)14.7 psi.
E)all of the above
A)760 mm Hg.
B)760 torr.
C)101,325 Pa.
D)14.7 psi.
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the equivalent pressure of 968 mm Hg in units of atm?
A)1.27 atm
B)0.785 atm
C)968 atm
D)1.30 atm
E)none of the above
A)1.27 atm
B)0.785 atm
C)968 atm
D)1.30 atm
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of following statements are consistent with the Kinetic Molecular Theory?
A)Gases are compressible because the volume of atoms is almost entirely open space.
B)Gases assume the shape and volume of their container because they are in constant,straight-line motion.
C)Gases have a low density because there is so much empty space between the particles.
D)Gas particles collide with each other and surfaces without losing any energy.
E)All of the above statements are consistent with the Kinetic Molecular Theory.
A)Gases are compressible because the volume of atoms is almost entirely open space.
B)Gases assume the shape and volume of their container because they are in constant,straight-line motion.
C)Gases have a low density because there is so much empty space between the particles.
D)Gas particles collide with each other and surfaces without losing any energy.
E)All of the above statements are consistent with the Kinetic Molecular Theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which one of the following is impossible for an ideal gas?
A)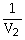
=
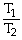
(
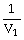
)
B)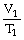
=
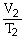
C)V2 = (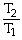
)V1
D)V1T1 = V2T2
E)none of the above
A)
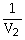
=
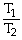
(
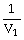
)
B)
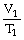
=
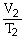
C)V2 = (
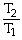
)V1
D)V1T1 = V2T2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
51
A balloon filled with 0.500 L of air at sea level is submerged in the water to a depth that produces a pressure of 3.25 atm.What is the volume of the balloon at this depth?
A)1.63 L
B)0.154 L
C)6.50 L
D)0.615 L
E)none of the above
A)1.63 L
B)0.154 L
C)6.50 L
D)0.615 L
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following is NOT part of the Kinetic Molecular Theory?
A)Gas particles do not repel each other.
B)There is a large distance between gas particles as compared to their relative size.
C)The size of the actual gas particles is small compared to the volume of the whole gas.
D)The average energy of the particles is dependent on the molecular mass of the particle.
E)All of the above statements are part of the Kinetic Molecular Theory.
A)Gas particles do not repel each other.
B)There is a large distance between gas particles as compared to their relative size.
C)The size of the actual gas particles is small compared to the volume of the whole gas.
D)The average energy of the particles is dependent on the molecular mass of the particle.
E)All of the above statements are part of the Kinetic Molecular Theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
53
All of the following statements are consistent with the kinetic molecular theory of gases EXCEPT
A)The size of the gas molecules is negligible compared to the total volume of the gas.
B)The average kinetic energy of the molecules of a gas is proportional to the temperature of the gas in kelvins.
C)The gas molecules collide with each other and with the surfaces around them.
D)Strong attractive forces hold the gas molecules together.
E)none of the above
A)The size of the gas molecules is negligible compared to the total volume of the gas.
B)The average kinetic energy of the molecules of a gas is proportional to the temperature of the gas in kelvins.
C)The gas molecules collide with each other and with the surfaces around them.
D)Strong attractive forces hold the gas molecules together.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
54
Charles's Law is expressed as:
A)V is proportional to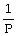
B)P is proportional to V
C)V is proportional to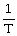
D)V is proportional to T
E)none of the above
A)V is proportional to
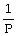
B)P is proportional to V
C)V is proportional to
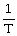
D)V is proportional to T
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following statements is TRUE for gases?
1.The temperature of a gas is inversely proportional to its pressure.
2.The volume of a gas is directly proportional to the pressure in torr.
3.The pressure of a gas is due to collisions of the gas molecules.
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)1 and 3 only
1.The temperature of a gas is inversely proportional to its pressure.
2.The volume of a gas is directly proportional to the pressure in torr.
3.The pressure of a gas is due to collisions of the gas molecules.
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)1 and 3 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
56
A barometer uses mercury because:
A)it is a convenient,safe,lightweight material.
B)the density of mercury is very large which allows the barometer to be short.
C)it is the traditional substance used,water could be as easily used.
D)it is the only liquid metal at room temperature.
E)All of the above are true.
A)it is a convenient,safe,lightweight material.
B)the density of mercury is very large which allows the barometer to be short.
C)it is the traditional substance used,water could be as easily used.
D)it is the only liquid metal at room temperature.
E)All of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
57
One liter of a gas is in a sealed chamber containing a moveable piston.If the piston is moved so that the volume of the gas is compressed to a volume of one-half liter,what will happen to the pressure on the gas? (Assume the temperature is constant and no gas particles are lost. )
A)The pressure will remain the same.
B)The pressure will be half of the original value.
C)The pressure will be twice the original value.
D)It would be impossible to move the piston since gases are not compressible.
E)none of the above
A)The pressure will remain the same.
B)The pressure will be half of the original value.
C)The pressure will be twice the original value.
D)It would be impossible to move the piston since gases are not compressible.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the equivalent pressure of 1520 torr in units of atm?
A)203,000
B)380.
C)2.00
D)1520
E)none of the above
A)203,000
B)380.
C)2.00
D)1520
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
59
Boyle's Law is expressed as:
A)V is proportional to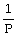
B)P is proportional to V
C)V is proportional to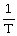
D)V is proportional to T
E)none of the above
A)V is proportional to
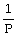
B)P is proportional to V
C)V is proportional to
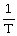
D)V is proportional to T
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the equivalent pressure of 0.905 atm in units of mm Hg?
A)688
B)840
C)0.905
D)13.3
E)none of the above
A)688
B)840
C)0.905
D)13.3
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
61
Suppose a balloon was released from the ground and rose to such a height that both the atmospheric pressure and atmospheric temperature decreased.Which statement is TRUE?
A)Both the temperature and pressure changes act to increase the balloon's volume.
B)Both the temperature and pressure changes act to decrease the balloon's volume.
C)The temperature change acts to increase the balloon's volume.
D)The pressure change acts to decrease the balloon's volume.
E)none of the above
A)Both the temperature and pressure changes act to increase the balloon's volume.
B)Both the temperature and pressure changes act to decrease the balloon's volume.
C)The temperature change acts to increase the balloon's volume.
D)The pressure change acts to decrease the balloon's volume.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the initial temperature (°C)of a system that has the pressure decreased by 10 times while the volume increased by 5 times with a final temperature of 150 K?
A)27
B)75
C)-198
D)300
E)none of the above
A)27
B)75
C)-198
D)300
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
63
Avogadro's Law is expressed as:
A)V is proportional to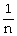
B)P is proportional to n
C)n is proportional to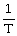
D)V is proportional to n
E)none of the above
A)V is proportional to
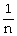
B)P is proportional to n
C)n is proportional to
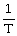
D)V is proportional to n
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
64
If the volume of a gas container at 32.0°C changes from 1.55 L to 755 mL,what will the final temperature be?
A)149°C
B)353°C
C)273°C
D)-124°C
E)none of the above
A)149°C
B)353°C
C)273°C
D)-124°C
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the initial temperature of a gas if the volume changed from 1.00 L to 1.10 L and the final temperature was determined to be 255.0°C?
A)480°C
B)-41°C
C)232°C
D)207°C
E)none of the above
A)480°C
B)-41°C
C)232°C
D)207°C
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
66
For an ideal gas,which of the following pairs of variables are inversely proportional to each other (if all other factors remain constant)?
A)P,V
B)P,T
C)V,T
D)n,P
E)none of the above
A)P,V
B)P,T
C)V,T
D)n,P
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the final volume of a 500.0 mL gas container that increased in temperature from 299 K to 333 K while the pressure increased from 1.00 atm to 1.54 atm?
A)0.691 L
B)2.77 L
C)0.362 L
D)1.45 L
E)none of the above
A)0.691 L
B)2.77 L
C)0.362 L
D)1.45 L
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
68
A gas sample occupies 3.50 liters of volume at 20.°C.What volume will this gas occupy at 100.°C (reported to three significant figures)?
A)0.224 L
B)2.75 L
C)4.46 L
D)17.5 L
E)none of the above
A)0.224 L
B)2.75 L
C)4.46 L
D)17.5 L
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
69
A balloon originally had a volume of 0.439 L at 44°C and a pressure of 729 torr.To what temperature must the balloon be cooled to reduce its volume to 378 mL if the pressure remained constant?
A)0°C
B)38°C
C)95°C
D)273°C
E)none of the above
A)0°C
B)38°C
C)95°C
D)273°C
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
70
If each of the following gas samples have the same temperature and pressure,which sample has the greatest volume?
A)1 gram of O2
B)1 gram of Ar
C)1 gram of H2
D)all have the same volume
E)not enough information
A)1 gram of O2
B)1 gram of Ar
C)1 gram of H2
D)all have the same volume
E)not enough information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
71
To solve problems using Avogadro's Law,which mathematical equation should be used?
A)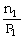
=
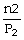
B)n1V1 = n2V2
C)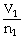
=
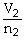
D)P1V1 = P2V2
E)none of the above
A)
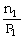
=
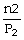
B)n1V1 = n2V2
C)
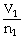
=
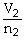
D)P1V1 = P2V2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
72
A sample of helium gas initially at 37.0°C,785 torr and 2.00 L was heated to 58.0°C while the volume expanded to 3.24 L.What is the final pressure in atm?
A)517
B)0.681
C)1.79
D)3.21
E)none of the above
A)517
B)0.681
C)1.79
D)3.21
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
73
A 325 mL sample of gas is initially at a pressure of 721 torr and a temperature of 32°C.If this gas is compressed to a volume of 286 mL and the pressure increases to 901 torr,what will be the new temperature of the gas (reported to three significant figures in °C)?
A)35.2°C
B)335°C
C)62.4°C
D)215°C
E)none of the above
A)35.2°C
B)335°C
C)62.4°C
D)215°C
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the proper form of the combined gas law?
A)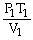
=
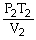
B)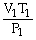
=
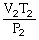
C)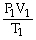
=
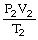
D)P1V1T1 = P2V2T2
E)none of the above
A)
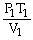
=
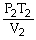
B)
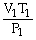
=
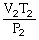
C)
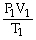
=
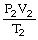
D)P1V1T1 = P2V2T2
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
75
A certain volume of gas was confined in a rigid container.If the pressure of the gas sample in the container was doubled,what happened to the temperature?
A)The temperature became one half.
B)The temperature doubled.
C)The temperature increased four times.
D)The temperature decreased one-third.
E)not enough information
A)The temperature became one half.
B)The temperature doubled.
C)The temperature increased four times.
D)The temperature decreased one-third.
E)not enough information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
76
If the initial pressure of a system was 1.00 atm and the volume was halved and the temperature was tripled,what is the final pressure?
A)2.00 atm
B)0.667 atm
C)1.50 atm
D)6.00 atm
E)not enough information
A)2.00 atm
B)0.667 atm
C)1.50 atm
D)6.00 atm
E)not enough information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
77
What is the final volume of a balloon that was initially 500.0 mL at 25°C and was then heated to 50°C?
A)461 mL
B)193 mL
C)1.00 L
D)542 mL
E)none of the above
A)461 mL
B)193 mL
C)1.00 L
D)542 mL
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
78
What happens to the volume of a gas when you double the number of moles of gas while keeping the temperature and pressure constant?
A)The volume is halved.
B)The volume doubles.
C)The volume decreases,but more information is needed.
D)The volume increases,but more information is needed.
E)none of the above
A)The volume is halved.
B)The volume doubles.
C)The volume decreases,but more information is needed.
D)The volume increases,but more information is needed.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
79
How many moles of gas were added to a balloon that started with 2.3 moles of gas and a volume of 1.4 L given that the final volume was 7.2 L?
A)9.5
B)4.4
C)12
D)0.085
E)none of the above
A)9.5
B)4.4
C)12
D)0.085
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck
80
Gas density can be calculated by dividing the mass of gas by its volume.If you took a balloon of gas and then warmed the balloon in a sunny window,what can now be said about the density of the gas in the balloon?
A)The gas density will remain the same.
B)The gas density will increase.
C)The gas density will decrease.
D)The density of gases is independent of temperature.
E)none of the above
A)The gas density will remain the same.
B)The gas density will increase.
C)The gas density will decrease.
D)The density of gases is independent of temperature.
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 112 في هذه المجموعة.
فتح الحزمة
k this deck








