Deck 8: Chemical Reactivity: Chemicals in Action
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/54
العب
ملء الشاشة (f)
Deck 8: Chemical Reactivity: Chemicals in Action
1
Which will have the largest molar mass?
A) 1 mol of Ar, argon
B) 1 mol of CO, carbon monoxide
C) 1 mol of N2, nitrogen
D) 1 mol of Sn, tin
A) 1 mol of Ar, argon
B) 1 mol of CO, carbon monoxide
C) 1 mol of N2, nitrogen
D) 1 mol of Sn, tin
1 mol of Sn, tin
2
Which is not a statement of the First Law of Thermodynamics?
A) Energy can be converted from one form to another.
B) Things are getting more screwed up every day.
C) There's no such thing as a free lunch.
D) You can't get something for nothing.
A) Energy can be converted from one form to another.
B) Things are getting more screwed up every day.
C) There's no such thing as a free lunch.
D) You can't get something for nothing.
Things are getting more screwed up every day.
3
What factors determine the rate of a reaction?
A) nature of reactants
B) concentration of reactants
C) activation energy
D) all of these
A) nature of reactants
B) concentration of reactants
C) activation energy
D) all of these
all of these
4
A catalyst speeds up a reaction rate because it
A) raises the reaction temperature.
B) provides an alternate lower energy pathway.
C) increases the energy of reactants.
D) removes products from the reaction mixture.
A) raises the reaction temperature.
B) provides an alternate lower energy pathway.
C) increases the energy of reactants.
D) removes products from the reaction mixture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
5
According to the second law of thermodynamics,
A) The quality of energy is conserved, but he quantity of energy is not conserved.
B) Every naturally occurring process produces some entropy.
C) Perpetual motion is possible.
D) Efficiencies of energy transformations are expected to be 100%.
A) The quality of energy is conserved, but he quantity of energy is not conserved.
B) Every naturally occurring process produces some entropy.
C) Perpetual motion is possible.
D) Efficiencies of energy transformations are expected to be 100%.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the molar mass of H2SO4?
A) 50 g
B) 49 g
C) 98 g
D) 7.0 g
A) 50 g
B) 49 g
C) 98 g
D) 7.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
7
What numbers are needed to balance the following equation, respectively? ____CO2 + ____H2O ____C6H12O6 + ____O2
A) 1, 1, 1, 1
B) 6, 6, 6, 6
C) 6, 6, 1, 6
D) 6, 1, 1, 6
A) 1, 1, 1, 1
B) 6, 6, 6, 6
C) 6, 6, 1, 6
D) 6, 1, 1, 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
8
A chemical reaction that releases heat during the reaction is
A) exothermic.
B) endothermic.
C) a source of quick heat.
D) impossible to reverse.
A) exothermic.
B) endothermic.
C) a source of quick heat.
D) impossible to reverse.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
9
For the equation, H2(g) + Cl2(g) 2 HCl(g), which statement is false?
A) 1 mol of Cl2(g) is a reactant.
B) 2 molecules of HCl are formed.
C) 4 g of H2(g) reacts with 1 mol of Cl2(g).
D) The sum of the coefficients of the reactants is 2.
A) 1 mol of Cl2(g) is a reactant.
B) 2 molecules of HCl are formed.
C) 4 g of H2(g) reacts with 1 mol of Cl2(g).
D) The sum of the coefficients of the reactants is 2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which is necessary for a chemical reaction to occur?
A) Reactant molecules must collide.
B) Collisions must be of a certain energy.
C) neither a nor b
D) both a and b
A) Reactant molecules must collide.
B) Collisions must be of a certain energy.
C) neither a nor b
D) both a and b

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
11
Exhibit 8-1
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. The atomic weights for Zn, H and Cl are 65.4 g, 1.0 g and 35.5 g, respectively. What is the mass for two moles of hydrochloric acid?
A) 36.5 g
B) 71.0 g
C) 35.5 g
D) 73.0 g
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. The atomic weights for Zn, H and Cl are 65.4 g, 1.0 g and 35.5 g, respectively. What is the mass for two moles of hydrochloric acid?
A) 36.5 g
B) 71.0 g
C) 35.5 g
D) 73.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
12
In all chemical changes,
A) reactants disappear completely.
B) all of the reactants must cease to exist.
C) the weight of products and reactants must balance.
D) products must be in the same physical state as the reactants.
A) reactants disappear completely.
B) all of the reactants must cease to exist.
C) the weight of products and reactants must balance.
D) products must be in the same physical state as the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
13
According to the first law of thermodynamics,
A) Energy is neither lost nor gained in an energy transformation.
B) Perpetual motion is possible.
C) Energy is not conserved.
D) Energy is being created as time passes.
A) Energy is neither lost nor gained in an energy transformation.
B) Perpetual motion is possible.
C) Energy is not conserved.
D) Energy is being created as time passes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
14
For the equation, 2 NaN3(s) 2 Na(s) + 3 N2(g), which statement is false?
A) The equation is balanced.
B) The products are sodium and nitrogen.
C) The sum of all coefficients is 7.
D) The products are all in the same state of matter.
A) The equation is balanced.
B) The products are sodium and nitrogen.
C) The sum of all coefficients is 7.
D) The products are all in the same state of matter.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
15
Preserving food by freezing controls reaction rates by
A) decreasing the number of collisions.
B) increasing concentration.
C) changing the number of effective reactant collisions.
D) Both a and c are correct.
A) decreasing the number of collisions.
B) increasing concentration.
C) changing the number of effective reactant collisions.
D) Both a and c are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
16
Exhibit 8-1
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. What weight of ZnCl2 will be produced from 1.00 mol of Zn?
A) 65.4 g
B) 98.9 g
C) 136 g
D) 71.0 g
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. What weight of ZnCl2 will be produced from 1.00 mol of Zn?
A) 65.4 g
B) 98.9 g
C) 136 g
D) 71.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
17
If 2 pounds of hydrogen react with chlorine to form 73 pounds of hydrogen chloride, what weight of chlorine must react with the 2 lb of hydrogen?
H2(g) + Cl2(g) 2 HCl(g)
A) 71 lb Cl2(g)
B) 35.5 lb Cl2(g)
C) 2 lb Cl2(g)
D) The weight of chlorine used depends on the reaction conditions.
H2(g) + Cl2(g) 2 HCl(g)
A) 71 lb Cl2(g)
B) 35.5 lb Cl2(g)
C) 2 lb Cl2(g)
D) The weight of chlorine used depends on the reaction conditions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
18
If you add more CO2(g) to an equilibrium mixture of CaCO3(s), CaO(s) and CO2(g) in a hot closed vessel, which way would you expect the equilibrium to shift ? CaCO3(s) 
CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
19
Exhibit 8-1
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. How many moles of hydrochloric acid react with one mole of zinc?
A) 1 mol
B) 2 mol
C) between 1 and 2 mol depending on how much hydrogen gas is formed
D) the same number as the number of moles of hydrogen produced
For the following questions, consider the equation below. Zinc will react with a solution of hydrochloric acid to produce hydrogen gas:
Zn(s) + 2 HCl(aq) ZnCl2(s) + H2(g)
-Refer to Exhibit 8-1. How many moles of hydrochloric acid react with one mole of zinc?
A) 1 mol
B) 2 mol
C) between 1 and 2 mol depending on how much hydrogen gas is formed
D) the same number as the number of moles of hydrogen produced

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which will increase the rate of a reaction?
A) increasing temperature
B) increasing temperature and concentration
C) increasing temperature and surface area
D) all of these
A) increasing temperature
B) increasing temperature and concentration
C) increasing temperature and surface area
D) all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is conserved when one balances a chemical equation?
A) molecules
B) atoms
C) products
D) reactants
A) molecules
B) atoms
C) products
D) reactants

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
22
How does changing concentration effect the rate of a chemical reaction?
A) Increasing the concentration of a reactant will increase the reaction rate.
B) The concentration has very little effect on reaction rates.
C) The reaction rate increases only when all reactant concentrations are increased.
D) Increasing the concentration of products increases reaction rates.
A) Increasing the concentration of a reactant will increase the reaction rate.
B) The concentration has very little effect on reaction rates.
C) The reaction rate increases only when all reactant concentrations are increased.
D) Increasing the concentration of products increases reaction rates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which is a statement of the Second Law of Thermodynamics?
A) Energy can be converted from one form to another.
B) Things are getting more screwed up every day.
C) There's no such thing as a free lunch.
D) You can't get something for nothing.
A) Energy can be converted from one form to another.
B) Things are getting more screwed up every day.
C) There's no such thing as a free lunch.
D) You can't get something for nothing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
24
The use of a fire blanket in extinguishing a clothing fire is most closely related to what factor relative to chemical reaction rates?
A) temperature of reaction medium
B) catalysis
C) equilibrium ratio of reactants and products
D) concentration of reactants
A) temperature of reaction medium
B) catalysis
C) equilibrium ratio of reactants and products
D) concentration of reactants

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
25
Chemical equilibrium exists for a chemical reaction when
A) equal amounts of chemicals exist on reactant and product sides.
B) one or more of the reactants are used completely.
C) all molecular activity stops.
D) a state of balance exists between two opposite changes occurring at the same rate.
A) equal amounts of chemicals exist on reactant and product sides.
B) one or more of the reactants are used completely.
C) all molecular activity stops.
D) a state of balance exists between two opposite changes occurring at the same rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many atoms are represented in the formula Ca3(PO4)2?
A) 9
B) 11
C) 13
D) 1
A) 9
B) 11
C) 13
D) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
27
Crumpling the pages of a newspaper to start a fire increases
A) surface contact.
B) reactant available.
C) equilibrium constant.
D) temperature effect on reaction rate.
A) surface contact.
B) reactant available.
C) equilibrium constant.
D) temperature effect on reaction rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the mass of one mole of water molecules, H2O? The atomic weight of hydrogen is 1 and the atomic weight of oxygen is 16.
A) 18 g H2O
B) 18 H2O
C) 6.02 1023 g H2O
D) 6.02 1023 H2O
A) 18 g H2O
B) 18 H2O
C) 6.02 1023 g H2O
D) 6.02 1023 H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
29
The three R's of waste prevention include
A) reuse products as much as possible.
B) recycle materials as much as possible.
C) reduce the amount of waste as much as possible.
D) all of the above
A) reuse products as much as possible.
B) recycle materials as much as possible.
C) reduce the amount of waste as much as possible.
D) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
30
If you remove some CO2(g) from an equilibrium mixture of CaCO3(s), CaO(s) and CO2(g) in a hot closed vessel, which way would you expect the equilibrium to shift ? CaCO3(s) 
CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
31
If you add more CaCO3(s) to an equilibrium mixture of CaCO3(s), CaO(s) and CO2(g) in a hot closed vessel, which way would you expect the equilibrium to shift ? CaCO3(s) 
CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

CaO(s) + CO2(g)
A) to form more reactant
B) to form more product
C) no change
D) indeterminable based in information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the sum of the coefficients when the following equation is balanced? Fe(s) + O2(g) Fe2O3(s)
A) 3
B) 6
C) 9
D) 10
A) 3
B) 6
C) 9
D) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
33
How many grams are in 2.40 mol of carbon tetrachloride?
A) 0.0156 g
B) 370 g
C) 114 g
D) 0.0506 g
A) 0.0156 g
B) 370 g
C) 114 g
D) 0.0506 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
34
What reaction rate factor is responsible for a dust explosion in a flour mill?
A) increased temperature
B) increased surface area
C) presence of catalysis
D) foreign chemicals
A) increased temperature
B) increased surface area
C) presence of catalysis
D) foreign chemicals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
35
Favorable chemical reactions
A) are generally explosive.
B) absorb energy from the surroundings.
C) occur only in the gaseous phase.
D) usually give off heat energy.
A) are generally explosive.
B) absorb energy from the surroundings.
C) occur only in the gaseous phase.
D) usually give off heat energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
36
How many moles are in 15.5 g of diphosphorous pentoxide?
A) 0.109 mol
B) 0.330 mol
C) 6.56 1023 mol
D) 2.20 103 mol
A) 0.109 mol
B) 0.330 mol
C) 6.56 1023 mol
D) 2.20 103 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the number of atoms in 1.0 mol of nitrogen dioxide, NO2?
A) 1.8 1024 atoms
B) 1.8 1023 atoms
C) 2.0 1023 atoms
D) 3 atoms
A) 1.8 1024 atoms
B) 1.8 1023 atoms
C) 2.0 1023 atoms
D) 3 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the coefficient of oxygen when the following molecular equation is correctly balanced? _____NH3(g) + _____O2(g) _____NO(g) + _____H2O(g)
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
39
Le Châtelier's principle says
A) equilibrium mixtures are changed only when reactants are added.
B) equilibrium mixtures will change to form more products when a reactant is added.
C) the balance between reactants and products is not altered by changing the product concentration.
D) equilibrium mixtures will not change when a stress is placed on the system.
A) equilibrium mixtures are changed only when reactants are added.
B) equilibrium mixtures will change to form more products when a reactant is added.
C) the balance between reactants and products is not altered by changing the product concentration.
D) equilibrium mixtures will not change when a stress is placed on the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following samples has the most entropy?
A) 1 mol solid hydrogen
B) 1 mol liquid hydrogen
C) 1 mol hydrogen gas
D) All are the same because they are all hydrogen.
A) 1 mol solid hydrogen
B) 1 mol liquid hydrogen
C) 1 mol hydrogen gas
D) All are the same because they are all hydrogen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
41
Consider the following two statements.
"Energy can be converted from one form to
another but cannot be destroyed nor created."
"The total amount of matter and
energy in the universe is constant."
Both of these are included in the first law of thermodynamics.
"Energy can be converted from one form to
another but cannot be destroyed nor created."
"The total amount of matter and
energy in the universe is constant."
Both of these are included in the first law of thermodynamics.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
42
Consider the following reaction.
PCl5(g) PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If the pressure is increased the forward reaction will be favored.
PCl5(g)
 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)If the pressure is increased the forward reaction will be favored.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
43
Consider the following energy profile for a reaction. 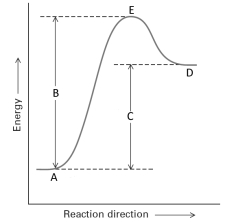 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
Ereaction is represented by the letter ________.
 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.Ereaction is represented by the letter ________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
44
An indicator, HIn, shows a color change from blue to yellow, depending on the acidity of the solution: HIn (aq) + H2O(l)  H3O+ (aq) + In- (aq)
H3O+ (aq) + In- (aq)
(blue) (yellow)
What color will the indicator be if vinegar is added to this solution?
A) blue
B) yellow
C) Addition of vinegar will have not effect on the indicator color.
 H3O+ (aq) + In- (aq)
H3O+ (aq) + In- (aq)(blue) (yellow)
What color will the indicator be if vinegar is added to this solution?
A) blue
B) yellow
C) Addition of vinegar will have not effect on the indicator color.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
45
Consider the following energy profile for a reaction.  Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
The reactants are represented by the letter _______.
 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.The reactants are represented by the letter _______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
46
Addition of a catalyst to a reaction and increasing the temperature of a reaction generally have the same effect on the reaction rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
47
In the following reaction, the products have lower entropy than the reactants.
CaCO3(s)
CaO(s) + CO2(g)
CaCO3(s)

CaO(s) + CO2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
48
The molar mass of sulfur trioxide is ________g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
49
The following energy profile represents an
exergonic reaction.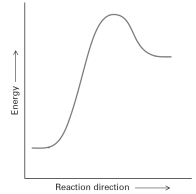
exergonic reaction.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
50
Consider the following energy profile for a reaction.  Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
If a catalyst were added to this reaction, the position of letter ______ would appear lower on the energy scale.
 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.If a catalyst were added to this reaction, the position of letter ______ would appear lower on the energy scale.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
51
The mass of 1 mol of Ar is smaller than the mass of 1 mol of CO2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
52
Consider the following energy profile for a reaction.  Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
Eact is represented by the letter_______.
 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.Eact is represented by the letter_______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
53
For a reaction to take place, the molecules that are reacting
A) must have more energy than the products.
B) must have less energy than the products.
C) must be able to reach to activation energy.
D) must be present in large numbers.
A) must have more energy than the products.
B) must have less energy than the products.
C) must be able to reach to activation energy.
D) must be present in large numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck
54
Consider the following energy profile for a reaction.  Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
The products of the reaction are presented by the letter________.
 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.The products of the reaction are presented by the letter________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 54 في هذه المجموعة.
فتح الحزمة
k this deck








