Deck 10: Oxidation-Reduction Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/47
العب
ملء الشاشة (f)
Deck 10: Oxidation-Reduction Reactions
1
Which of the batteries listed below is a reusable battery?
A) dry cell battery
B) mercury battery
C) alkaline battery
D) lead storage battery
A) dry cell battery
B) mercury battery
C) alkaline battery
D) lead storage battery
lead storage battery
2
When a zinc strip is placed in a copper(II) sulfate solution,
A) the copper(II) solution will lose its blue color.
B) the zinc will dissolve.
C) black crystals of copper atoms appear.
D) All of the above occur.
A) the copper(II) solution will lose its blue color.
B) the zinc will dissolve.
C) black crystals of copper atoms appear.
D) All of the above occur.
All of the above occur.
3
Charge is transported from place to place in a battery by
A) an anode.
B) a cathode.
C) the migration of ions.
D) an external electrical circuit.
A) an anode.
B) a cathode.
C) the migration of ions.
D) an external electrical circuit.
the migration of ions.
4
Which of the following equations represents the reduction of carbon?
A) C + 2 H2 CH4
B) C + O2 CO2
C) 2 C + O2 2 CO
D) C2+ C4+ + 2e-
A) C + 2 H2 CH4
B) C + O2 CO2
C) 2 C + O2 2 CO
D) C2+ C4+ + 2e-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following equations does not fit the definition of oxidation?
A) Ag+ + e- Ag
B) C2H6 H2 + C2H4
C) C + O2 CO2
D) Na Na+ + e-
A) Ag+ + e- Ag
B) C2H6 H2 + C2H4
C) C + O2 CO2
D) Na Na+ + e-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
6
In the equation 2 Na(s) + Cl2(g) 2 Na+ + 2 Cl-, the oxidizing agent is
A) Na(s).
B) Cl2(g).
C) Na+.
D) Cl-.
A) Na(s).
B) Cl2(g).
C) Na+.
D) Cl-.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following is not necessary for corrosion of iron?
A) iron
B) water
C) light
D) oxygen
A) iron
B) water
C) light
D) oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
8
In a fuel cell using oxygen as the oxidizer and hydrogen as the fuel, what is the overall cell reaction?
A) H2O H2 + O2
B) 2 H2 + O2 2 H2O
C) H2O + 4 e- 2 H2 + 4 OH-
D) O2 + 4 H+ + 4 e- 2 H2O
A) H2O H2 + O2
B) 2 H2 + O2 2 H2O
C) H2O + 4 e- 2 H2 + 4 OH-
D) O2 + 4 H+ + 4 e- 2 H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
9
A battery which may be recharged is called a(n)
A) primary battery.
B) dry cell.
C) electroplating cell.
D) secondary battery.
A) primary battery.
B) dry cell.
C) electroplating cell.
D) secondary battery.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
10
PbSO4, Pb, and H2SO4 are chemicals in
A) dry cell
B) primary battery
C) salt bridge
D) automobile battery
A) dry cell
B) primary battery
C) salt bridge
D) automobile battery

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
11
Antioxidant vitamins
A) react with DNA.
B) donate electrons to free radicals.
C) are reduced.
D) form free radicals in the body.
A) react with DNA.
B) donate electrons to free radicals.
C) are reduced.
D) form free radicals in the body.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following might prevent corrosion of iron?
A) a water spray
B) a coating of zinc
C) acid treatments
D) salt solutions
A) a water spray
B) a coating of zinc
C) acid treatments
D) salt solutions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
13
In the equation SnO2(g) + 2 C(s) Sn(s) + 2 CO(g), what substance is the reducing agent?
A) SnO2
B) C
C) Sn
D) CO
A) SnO2
B) C
C) Sn
D) CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
14
In an electrolysis reaction, oxidation occurs at which electrode?
A) cathode
B) anode
C) both electrodes
D) neither electrode
A) cathode
B) anode
C) both electrodes
D) neither electrode

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following is not a definition of oxidation?
A) loss of electrons
B) loss of hydrogen
C) loss of protons
D) addition of oxygen
A) loss of electrons
B) loss of hydrogen
C) loss of protons
D) addition of oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which one of the following will not combine with oxygen?
A) nitrogen
B) aluminum
C) hydrogen
D) helium
A) nitrogen
B) aluminum
C) hydrogen
D) helium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
17
In a lead storage battery, what lead compound is formed at both the cathode and the anode during discharge?
A) lead oxide (PbO2)
B) lead chloride (PbCl2)
C) lead sulfate (PbSO4)
D) lead oxide (PbO)
A) lead oxide (PbO2)
B) lead chloride (PbCl2)
C) lead sulfate (PbSO4)
D) lead oxide (PbO)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
18
Free radicals are particles that
A) have an unpaired electron.
B) act as oxidizing agents.
C) grab another electron from a neighboring molecule.
D) all of the above
A) have an unpaired electron.
B) act as oxidizing agents.
C) grab another electron from a neighboring molecule.
D) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which is not an example of a primary battery?
A) lead-acid automobile battery
B) alkaline battery
C) mercury battery
D) lithium battery
A) lead-acid automobile battery
B) alkaline battery
C) mercury battery
D) lithium battery

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the batteries listed below is a primary battery?
A) lithium ion battery
B) alkaline battery
C) nickel-cadmium battery
D) lead acid battery
A) lithium ion battery
B) alkaline battery
C) nickel-cadmium battery
D) lead acid battery

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
21
Free radicals which are also oxidizing agents do not occur naturally in the human body.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following are paired correctly?
A) H2O2 - oxidizing agent
B) NaOCl - reducing agent
C) Cl2 - reducing agent
D) SO2 - oxidizing agent
A) H2O2 - oxidizing agent
B) NaOCl - reducing agent
C) Cl2 - reducing agent
D) SO2 - oxidizing agent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following is the function of a bleaching agent?
A) oxidize colored chemicals
B) reduce colored chemicals
C) oxidize bleach
D) remove salts from hard water
A) oxidize colored chemicals
B) reduce colored chemicals
C) oxidize bleach
D) remove salts from hard water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following is a free radical?
A) SO2
B) SO3
C) NO
D) N2O
A) SO2
B) SO3
C) NO
D) N2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider the following image, showing on the atomic level, the reaction occurring when a Zn wire is placed in a copper(II) nitrate solution. 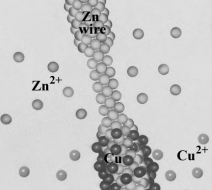 Which of the following correctly describes the image?
Which of the following correctly describes the image?
A) Zinc is being oxidized.
B) Copper is being reduced.
C) Zinc is the reducing agent.
D) All of the above are correct descriptions.
 Which of the following correctly describes the image?
Which of the following correctly describes the image?A) Zinc is being oxidized.
B) Copper is being reduced.
C) Zinc is the reducing agent.
D) All of the above are correct descriptions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
26
The reaction(s) found in primary batteries are easily reversible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which molecule would be a good oxidizing agent?
A) H2
B) SO2
C) C
D) H2O2
A) H2
B) SO2
C) C
D) H2O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
28
H2 is a often oxidized and O2 is often an oxidizing agent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
29
Electrolysis
A) is the process of producing electricity.
B) uses electrical energy to produce chemical change.
C) is used to reduce aluminum ore to pure metal.
D) both b and c
A) is the process of producing electricity.
B) uses electrical energy to produce chemical change.
C) is used to reduce aluminum ore to pure metal.
D) both b and c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
30
Vitamin C and beta-carotene are antioxidants found currently in vitamin supplements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
31
Reduction can be viewed as a either a gain of electrons or hydrogen atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
32
Identify the oxidized species in the following equation 4Al(s) + 3O2(g) 2Al2O3(s)
A) Al(s)
B) O2(g)
C) Al3+
D) O2-
A) Al(s)
B) O2(g)
C) Al3+
D) O2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which molecule would be a good reducing agent?
A) O2
B) O3
C) H2
D) H2O2
A) O2
B) O3
C) H2
D) H2O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following is not an antioxidant vitamin?
A) vitamin A
B) vitamin D
C) vitamin C
D) vitamin E
A) vitamin A
B) vitamin D
C) vitamin C
D) vitamin E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
35
Consider the reaction: 3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
Which of the following statements is true about Fe2+?
A) Fe2+ is losing electrons.
B) Fe2+ is oxidized.
C) Fe2+ is the oxidizing agent.
D) Fe2+ is the reducing agent.
Which of the following statements is true about Fe2+?
A) Fe2+ is losing electrons.
B) Fe2+ is oxidized.
C) Fe2+ is the oxidizing agent.
D) Fe2+ is the reducing agent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following is a free radical?
A) ozone, O3
B) fluorine, F2
C) chlorine, Cl2
D) nitrogen dioxide, NO2
A) ozone, O3
B) fluorine, F2
C) chlorine, Cl2
D) nitrogen dioxide, NO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
37
Reducing agents gain electrons and are oxidized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
38
The substance oxidized in an oxidation reduction reaction serves as the reducing agent by losing electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
39
Identify the reduced species in the following equation CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
A) C
B) O2
C) H
D) H2O
A) C
B) O2
C) H
D) H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
40
Oxidation always occurs
A) with the loss of electrons.
B) with the gain of electrons.
C) with the gain of hydrogen.
D) only for metal atoms and not ions.
A) with the loss of electrons.
B) with the gain of electrons.
C) with the gain of hydrogen.
D) only for metal atoms and not ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
41
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.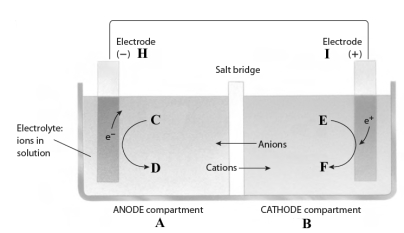
Complete the following questions using the letters shown in the image.
-Oxidation occurs in compartment _____.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-Oxidation occurs in compartment _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
42
The reducing agent in the following reaction is ___________.
I2 + Zn 2I- + Zn2+
I2 + Zn 2I- + Zn2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
43
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.
Complete the following questions using the letters shown in the image.
-The Fe2+ should be placed at letter ____.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-The Fe2+ should be placed at letter ____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
44
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.
Complete the following questions using the letters shown in the image.
-The electrons flow from electrode _____ to electrode _____.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-The electrons flow from electrode _____ to electrode _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
45
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.
Complete the following questions using the letters shown in the image.
-Al should be placed at letter _____.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-Al should be placed at letter _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
46
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.
Complete the following questions using the letters shown in the image.
-If the electrolyte contains nitrate ions, they will move from compartment ___ to compartment ___.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-If the electrolyte contains nitrate ions, they will move from compartment ___ to compartment ___.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
47
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.
Complete the following questions using the letters shown in the image.
-The oxidizing agent is found in compartment ______.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below.

Complete the following questions using the letters shown in the image.
-The oxidizing agent is found in compartment ______.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck








