Deck 9: Models of Chemical Bonding
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/75
العب
ملء الشاشة (f)
Deck 9: Models of Chemical Bonding
1
The diameter of a chloride ion is 362 pm, and the diameter of a potassium ion is 276 pm. What is the distance between the nuclei of adjacent chloride and potassium ions in solid potassium chloride?
A) 1276 pm
B) 638 pm
C) 319 pm
D) 181 pm
E) 138 pm
A) 1276 pm
B) 638 pm
C) 319 pm
D) 181 pm
E) 138 pm
319 pm
2
A Born-Haber cycle applied to the formation reaction of an ionic solid
A) is normally used to calculate ionization energy.
B) is normally used to calculate electron affinity.
C) is normally used to calculate bond energy.
D) is normally used to determine the overall enthalpy change.
E) is an application of Hess's Law.
A) is normally used to calculate ionization energy.
B) is normally used to calculate electron affinity.
C) is normally used to calculate bond energy.
D) is normally used to determine the overall enthalpy change.
E) is an application of Hess's Law.
is an application of Hess's Law.
3
The lattice energy for ionic crystals increases as the charge on the ions _____________ and the size of the ions __________________.
A) increases, increases
B) increases, decreases
C) decreases, increases
D) decreases, decreases
E) None of the above is generally correct.
A) increases, increases
B) increases, decreases
C) decreases, increases
D) decreases, decreases
E) None of the above is generally correct.
increases, decreases
4
Select the correct formula for a compound formed from barium and nitrogen.
A) BaN
B) BaN2
C) Ba2N3
D) Ba2N
E) Ba3N2
A) BaN
B) BaN2
C) Ba2N3
D) Ba2N
E) Ba3N2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
5
Select the compound with the lowest (i.e., least negative) lattice energy.
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
6
Select the element whose Lewis symbol is correct.
A)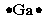
B)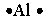
C)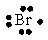
D)
E)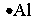
A)
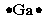
B)
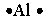
C)
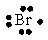
D)

E)
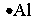

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following contains ionic bonding?
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following contains covalent bonds?
A) BaO
B) IBr
C) Mg
D) LiBr
E) Cu
A) BaO
B) IBr
C) Mg
D) LiBr
E) Cu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
9
The lattice energy of CaF2 is the energy change for which one, if any, of the following processes?
A) Ca2+(s) + 2F-(g) CaF2(g)
B) CaF2(g) CaF2(s)
C) Ca(g) + 2F(g) CaF2(s)
D) CaF2(aq) CaF2(s)
E) none of the above
A) Ca2+(s) + 2F-(g) CaF2(g)
B) CaF2(g) CaF2(s)
C) Ca(g) + 2F(g) CaF2(s)
D) CaF2(aq) CaF2(s)
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
10
Select the correct formula for a compound formed from calcium and chlorine.
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
11
Select the element whose Lewis symbol is correct.
A)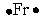
B)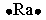
C)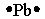
D)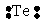
E)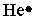
A)
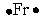
B)
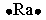
C)
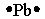
D)
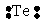
E)
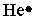

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
12
Select the compound with the highest (i.e., most negative) lattice energy.
A) CaS(s)
B) BaO(s)
C) NaI(s)
D) LiBr(s)
E) MgO(s)
A) CaS(s)
B) BaO(s)
C) NaI(s)
D) LiBr(s)
E) MgO(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
13
In which of these substances are the atoms held together by polar covalent bonding?
A) SrCl2
B) CsCl
C) ClF
D) TiF2
E) S8
A) SrCl2
B) CsCl
C) ClF
D) TiF2
E) S8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is an ionic compound?
A) H2S
B) NH3
C) I2
D) KI
E) CCl4
A) H2S
B) NH3
C) I2
D) KI
E) CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following is a covalent compound?
A) Na2O
B) CaCl2
C) Cl2O
D) CsCl
E) Al2O3
A) Na2O
B) CaCl2
C) Cl2O
D) CsCl
E) Al2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
16
In which of these substances are the atoms held together by metallic bonding?
A) CO2
B) Si
C) Br2
D) S8
E) Cr
A) CO2
B) Si
C) Br2
D) S8
E) Cr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
17
kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
17
For which of the following elements (in their normal, stable, forms) would it be correct to describe the bonding as involving "electron pooling"?
A) hydrogen
B) helium
C) sulfur
D) iodine
E) aluminum
A) hydrogen
B) helium
C) sulfur
D) iodine
E) aluminum

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
18
The lattice energy of MgCl2 is the energy change for which one of the following processes?
A) Mg(s) + Cl2(g) MgCl2(s)
B) Mg(g) + 2Cl(g) MgCl2(s)
C) Mg2+(s) + 2Cl-(g) gCl2(g)
D) Mg2+(g) + 2Cl-(g) MgCl2(s)
E) MgCl2(aq) MgCl2(s)
A) Mg(s) + Cl2(g) MgCl2(s)
B) Mg(g) + 2Cl(g) MgCl2(s)
C) Mg2+(s) + 2Cl-(g) gCl2(g)
D) Mg2+(g) + 2Cl-(g) MgCl2(s)
E) MgCl2(aq) MgCl2(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
19
Calculate the lattice energy of magnesium sulfide from the data given below.
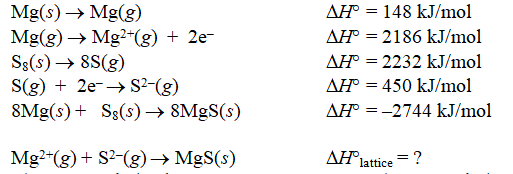
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D)3406 kJ/mol
E)none of the above
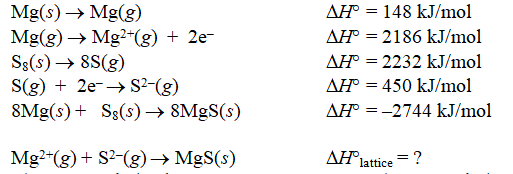
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D)3406 kJ/mol
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
20
Analysis of an unknown substance showed that it has a high boiling point and is brittle. It is an insulator as a solid but conducts electricity when melted. Which of the following substances would have those characteristics?
A) HCl
B) Al
C) KBr
D) SiF4
E) I2
A) HCl
B) Al
C) KBr
D) SiF4
E) I2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
21
Arrange calcium, rubidium, sulfur, and arsenic in order of decreasing electronegativity.
A) S > As > Rb > Ca
B) S > As > Ca > Rb
C) As > S > Rb > Ca
D) As > S > Ca > Rb
E) None of the above orders is correct.
A) S > As > Rb > Ca
B) S > As > Ca > Rb
C) As > S > Rb > Ca
D) As > S > Ca > Rb
E) None of the above orders is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
22
Combustion of a fat will release more energy than combustion of an equal mass of carbohydrate because
A) fats contain more bonds to oxygen than carbohydrates.
B) fats contain fewer bonds to oxygen than carbohydrates.
C) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the carbon-oxygen and oxygen-hydrogen bonds in the reaction products (carbon dioxide and water).
D) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the bonds in carbohydrates.
E) fats have higher molar masses than carbohydrates.
A) fats contain more bonds to oxygen than carbohydrates.
B) fats contain fewer bonds to oxygen than carbohydrates.
C) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the carbon-oxygen and oxygen-hydrogen bonds in the reaction products (carbon dioxide and water).
D) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the bonds in carbohydrates.
E) fats have higher molar masses than carbohydrates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
23
Arrange aluminum, nitrogen, phosphorus, and indium in order of increasing electronegativity.
A) Al < In < N < P
B) Al < In < P < N
C) In < Al < P < N
D) In < P < Al < N
E) None of the above orders is correct.
A) Al < In < N < P
B) Al < In < P < N
C) In < Al < P < N
D) In < P < Al < N
E) None of the above orders is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
24
Based on electronegativity trends in the periodic table, predict which of the following compounds will have the greatest % ionic character in its bonds.
A) H2O
B) LiI
C) CaO
D) RbF
E) HCl
A) H2O
B) LiI
C) CaO
D) RbF
E) HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following elements is the most electronegative?
A) S
B) Ru
C) Si
D) Te
E) Cs
A) S
B) Ru
C) Si
D) Te
E) Cs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
26
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change H° for the hydrogenation of ethyne (acetylene) to ethane. 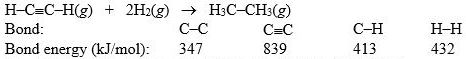
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ
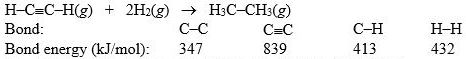
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
27
When two atoms form a covalently-bonded diatomic molecule, the distance between the nuclei at which the potential energy is at a minimum is called
A) the bond energy.
B) the bond length.
C) the molecular diameter.
D) the covalent radius.
E) the covalent diameter.
A) the bond energy.
B) the bond length.
C) the molecular diameter.
D) the covalent radius.
E) the covalent diameter.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
28
Arrange the following bonds in order of increasing bond strength.
A) C-I < C-Br < C-Cl < C-F
B) C-F < C-Cl < C-Br < C-I
C) C-Br < C-I < C-Cl < C-F
D) C-I < C-Br < C-F< C-Cl
E) None of the above orders is correct.
A) C-I < C-Br < C-Cl < C-F
B) C-F < C-Cl < C-Br < C-I
C) C-Br < C-I < C-Cl < C-F
D) C-I < C-Br < C-F< C-Cl
E) None of the above orders is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
29
Quartz (SiO2) is a solid with a melting point of 1550 °C. The bonding in quartz is best described as
A) lattice energy.
B) network attractions.
C) ionic bonding.
D) covalent bonding.
E) metallic bonding.
A) lattice energy.
B) network attractions.
C) ionic bonding.
D) covalent bonding.
E) metallic bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which one of the following properties is least characteristic of typical ionic compounds?
A) high melting point
B) high boiling point
C) brittleness
D) poor electrical conductor when solid
E) poor electrical conductor when molten
A) high melting point
B) high boiling point
C) brittleness
D) poor electrical conductor when solid
E) poor electrical conductor when molten

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
31
Nitrogen and hydrogen combine to form ammonia in the Haber process. Calculate (in kJ) the standard enthalpy change H° for the reaction written below, using the bond energies given. 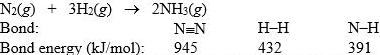
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ
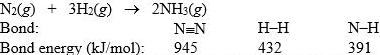
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following elements is the most electronegative?
A) Ne
B) Rb
C) P
D) I
E) Cl
A) Ne
B) Rb
C) P
D) I
E) Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
33
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 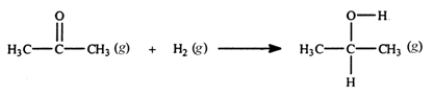

A) -484 kJ
B) -366 kJ
C) -48 kJ
D) +48 kJ
E) +366 kJ


A) -484 kJ
B) -366 kJ
C) -48 kJ
D) +48 kJ
E) +366 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
34
Select the strongest bond in the following group.
A) C-S
B) C-O
C) C=C
D) C N
E) C-F
A) C-S
B) C-O
C) C=C
D) C N
E) C-F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
35
Electronegativity is a measure of
A) the energy needed to remove an electron from an atom.
B) the energy released when an electron is added to an atom.
C) the magnitude of the negative charge on an electron.
D) the attraction by an atom for electrons in a chemical bond.
E) the magnitude of the negative charge on a molecule.
A) the energy needed to remove an electron from an atom.
B) the energy released when an electron is added to an atom.
C) the magnitude of the negative charge on an electron.
D) the attraction by an atom for electrons in a chemical bond.
E) the magnitude of the negative charge on a molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which one of the following properties is least characteristic of substances composed of small, covalently-bonded molecules?
A) low melting point
B) low boiling point
C) weak bonds
D) poor electrical conductor when solid
E) poor electrical conductor when molten
A) low melting point
B) low boiling point
C) weak bonds
D) poor electrical conductor when solid
E) poor electrical conductor when molten

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
37
Using the bond energies provided below, calculate H° for the reaction CH4(g) + 4Cl2(g) CCl4(g) + 4HCl(g)
Bond energies: C-H = 413 kJ/mol, Cl-Cl = 243 kJ/mol, C-Cl = 339 kJ/mol, H-Cl = 427 kJ/mol
A) 1422 kJ
B) 440 kJ
C) 110 kJ
D) -110 kJ
E) - 440 kJ
Bond energies: C-H = 413 kJ/mol, Cl-Cl = 243 kJ/mol, C-Cl = 339 kJ/mol, H-Cl = 427 kJ/mol
A) 1422 kJ
B) 440 kJ
C) 110 kJ
D) -110 kJ
E) - 440 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
38
Arrange oxygen, sulfur, calcium, rubidium, and potassium in order of decreasing electronegativity.
A) O > S > Ca > K > Rb
B) O > S > Ca > Rb > K
C) O > S > Rb > K > Ca
D) O > S > Rb > Ca > K
E) None of the above orders is correct.
A) O > S > Ca > K > Rb
B) O > S > Ca > Rb > K
C) O > S > Rb > K > Ca
D) O > S > Rb > Ca > K
E) None of the above orders is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
39
When one mole of each of the following liquids is burned, which will produce the most heat energy?
A) C6H14
B) C5H12
C) C6H14O
D) C6H12O
E) C6H10O3
A) C6H14
B) C5H12
C) C6H14O
D) C6H12O
E) C6H10O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following elements is the least electronegative?
A) Si
B) Se
C) S
D) Sc
E) Sr
A) Si
B) Se
C) S
D) Sc
E) Sr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
41
Ionic bonding typically occurs when a ______________ bonds with a ______________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
42
A hypothetical ionic substance will not form merely because it has a high lattice energy. Explain why, using energy-based arguments.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
43
Most of the copper sold in major metal markets is highly purified, typically to 99.99%. Why is this?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
44
Analysis of an unknown substance showed that it has a moderate melting point and is a good conductor of heat and electricity in the solid phase. Which of the following substances would have those characteristics?
A) NaCl
B) Si
C) CCl4
D) I2
E) Ga
A) NaCl
B) Si
C) CCl4
D) I2
E) Ga

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following compounds displays the greatest ionic character in its bonds?
A) NO2
B) CO2
C) H2O
D) HF
E) NH3
A) NO2
B) CO2
C) H2O
D) HF
E) NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
46
Covalent bonding typically occurs when a ______________ bonds with a ______________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
47
The melting points of metals are only moderately high because
A) metallic bonding is weak.
B) metals have fewer bonding electrons than non-metals.
C) metals also have relatively low boiling points.
D) the melting process does not break the metallic bonds.
E) metals prefer to be bonded to non-metals.
A) metallic bonding is weak.
B) metals have fewer bonding electrons than non-metals.
C) metals also have relatively low boiling points.
D) the melting process does not break the metallic bonds.
E) metals prefer to be bonded to non-metals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following period 3 chlorides would be expected to have the highest melting point?
A) MgCl2
B) AlCl3
C) SiCl4
D) PCl3
E) SCl2
A) MgCl2
B) AlCl3
C) SiCl4
D) PCl3
E) SCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
49
In not more than three sentences, describe the key features of bonding in solid aluminum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
50
When an atom is represented in a Lewis electron dot symbol, the element symbol represents ______________ and the dots represent ______________.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
51
Describe, with appropriate explanations, the key factors which affect the magnitude of the lattice energy of an ionic substance.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
52
Describe in brief how electronegativity values can be used to predict the percent ionic character of a bond between two atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
53
Using appropriate, real examples to illustrate your answer, describe the correlation between bond energy and bond length for a series of single bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
54
The lattice energy of rubidium chloride is the energy change accompanying the process
Rb+(g) + Cl-(g) RbCl(s)
Calculate the lattice energy of RbCl using the following data: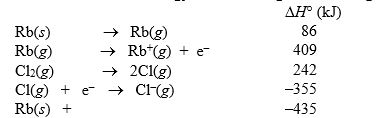
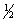 Cl2(g) RbCl(s)
Cl2(g) RbCl(s)
Rb+(g) + Cl-(g) RbCl(s)
Calculate the lattice energy of RbCl using the following data:
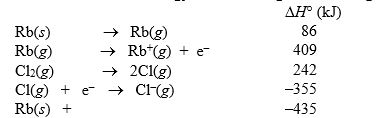
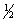 Cl2(g) RbCl(s)
Cl2(g) RbCl(s) 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
55
In not more than three sentences, describe the electron arrangement responsible for bonding in solid SrCl2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
56
Using appropriate, real examples to illustrate your answer, describe the correlation between bond energy and bond length for a series of varying bond order.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which one of the following properties is least characteristic of typical metals?
A) moderately high melting point
B) high boiling point
C) brittleness
D) good electrical conductor when solid
E) good electrical conductor when molten
A) moderately high melting point
B) high boiling point
C) brittleness
D) good electrical conductor when solid
E) good electrical conductor when molten

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
58
Select the most polar bond amongst the following.
A) C-O
B) Si-F
C) Cl-F
D) C-F
E) C-I
A) C-O
B) Si-F
C) Cl-F
D) C-F
E) C-I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
59
In not more than three sentences, describe the electron arrangement responsible for bonding in Cl2 molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
60
Give a clear and concise definition of the term "electronegativity"; i.e., what does it measure?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
61
Electronegativities on Pauling's scale are calculated from ionization energies and electron affinities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
62
The lattice energy of large ions is greater in magnitude than that of small ions of the same charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
63
The majority of elements are good electrical conductors when in solid form.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
64
The lattice energy is the energy released when separated ions in the gas phase combine to form ionic molecules in the gas phase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
65
Covalently bonded substances do not necessarily exist as separate molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
66
The stronger the bonds in a fuel, the more energy it will yield.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
67
No real bonds are 100% ionic in character.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
68
A single covalent bond consists of a single delocalized electron pair.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
69
The electrostatic energy of two charged particles is inversely proportional to the distance between them.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
70
The more C-O and O-H bonds there are in a substance, the greater will be the amount of heat released when a fixed mass of the substance is burned.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
71
The electrostatic energy of two charged particles is inversely proportional to the square of the distance between them.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
72
As a measure of the strength of metallic bonding, the boiling point of a metal is a better indicator than its melting point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
73
Bond energy increases as bond order increases, for bonding between a given pair of atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
74
In covalent bond formation, the potential energy reaches a maximum when the internuclear distance is equal to the bond length.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck








