Deck 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/111
العب
ملء الشاشة (f)
Deck 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
1
Octane is a component of fuel used in internal combustion engines. The dominant intermolecular forces in octane are
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonds.
E) carbon-hydrogen bonds.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonds.
E) carbon-hydrogen bonds.
London dispersion forces.
2
Liquid sodium can be used as a heat transfer fluid. Its vapor pressure is 40.0 torr at 633°C and 400.0 torr at 823°C. Calculate its heat of vaporization.
A) 43.4 kJ/mol
B) 52.5 kJ/mol
C) 70.6 kJ/mol
D) 1.00 × 102 kJ/mol
E) none of the above
A) 43.4 kJ/mol
B) 52.5 kJ/mol
C) 70.6 kJ/mol
D) 1.00 × 102 kJ/mol
E) none of the above
1.00 × 102 kJ/mol
3
Which of the following is true about kinetic energy, Ek, and potential energy, Ep, when ethyl alcohol at 40°C is compared with ethyl alcohol at 20°C?
A) Ek(40°C) < Ek(20°C); Ep(40°C) Ep(20°C)
B) Ek(40°C) > Ek(20°C); Ep(40°C) Ep(20°C)
C) Ep(40°C) < Ep(20°C); Ek(40°C) Ek(20°C)
D) Ep(40°C) > Ep(20°C); Ek(40°C) Ek(20°C)
E) Ep(40°C) > Ep(20°C); Ek(40°C) > Ek(20°C)
A) Ek(40°C) < Ek(20°C); Ep(40°C) Ep(20°C)
B) Ek(40°C) > Ek(20°C); Ep(40°C) Ep(20°C)
C) Ep(40°C) < Ep(20°C); Ek(40°C) Ek(20°C)
D) Ep(40°C) > Ep(20°C); Ek(40°C) Ek(20°C)
E) Ep(40°C) > Ep(20°C); Ek(40°C) > Ek(20°C)
Ek(40°C) > Ek(20°C); Ep(40°C) Ep(20°C)
4
The Clausius-Clapeyron equation is used in calculations of
A) melting and freezing points.
B) vapor pressures of liquids.
C) osmotic pressures of solutions.
D) heats of vaporization at different temperatures.
E) crystal structure.
A) melting and freezing points.
B) vapor pressures of liquids.
C) osmotic pressures of solutions.
D) heats of vaporization at different temperatures.
E) crystal structure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
5
Ammonia's unusually high melting point is the result of
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) ionic bonding.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) ionic bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
6
Examine the following phase diagram and identify the feature represented by point A. 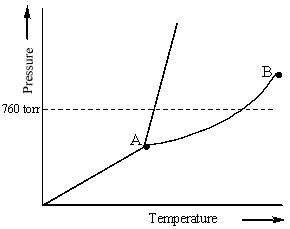
A) melting point
B) critical point
C) triple point
D) sublimation point
E) boiling point

A) melting point
B) critical point
C) triple point
D) sublimation point
E) boiling point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
7
The phase diagram for xenon has a solid-liquid curve with a positive slope. Which of the following is true?
A) Solid xenon has a higher density than liquid xenon.
B) Solid xenon has the same density as liquid xenon.
C) The phase diagram cannot be used to predict which phase of xenon is denser.
D) Freezing xenon is an endothermic process.
E) None of the above statements is true.
A) Solid xenon has a higher density than liquid xenon.
B) Solid xenon has the same density as liquid xenon.
C) The phase diagram cannot be used to predict which phase of xenon is denser.
D) Freezing xenon is an endothermic process.
E) None of the above statements is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
8
In hydrogen iodide __________________ are the most important intermolecular forces.
A) dipole-dipole forces
B) London dispersion forces
C) hydrogen bonding
D) covalent bonds
E) polar covalent bonds
A) dipole-dipole forces
B) London dispersion forces
C) hydrogen bonding
D) covalent bonds
E) polar covalent bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following quantities is generally not obtainable from a single heating or cooling curve of a substance, measured at atmospheric pressure?
A) melting point
B) boiling point
C) triple point
D) heat of fusion
E) heat of vaporization
A) melting point
B) boiling point
C) triple point
D) heat of fusion
E) heat of vaporization

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
10
A 5.00 g sample of water vapor, initially at 155°C is cooled at atmospheric pressure, producing ice at -55°C. Calculate the amount of heat energy lost by the water sample in this process, in kJ. Use the following data: specific heat capacity of ice is 2.09 J/g.K; specific heat capacity of liquid water is 4.18 J/g.K; specific heat capacity of water vapor is 1.84 J/g.K; heat of fusion of ice is 336 J/g; heat of vaporization of water is 2260 J/g.
A) 15.6 kJ
B) 10.2 kJ
C) 5.4 kJ
D) 3.2 kJ
E) 1.6 kJ
A) 15.6 kJ
B) 10.2 kJ
C) 5.4 kJ
D) 3.2 kJ
E) 1.6 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
11
Examine the following phase diagram and determine what phase exists at point F. 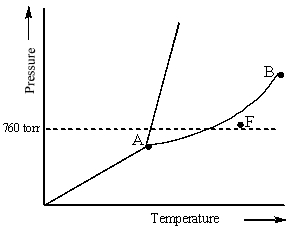
A) vapor + liquid
B) vapor
C) liquid
D) solid
E) supercritical fluid

A) vapor + liquid
B) vapor
C) liquid
D) solid
E) supercritical fluid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
12
Examine the following phase diagram and identify the feature represented by point B. 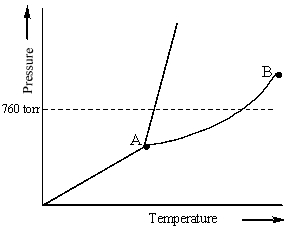
A) melting point
B) triple point
C) critical point
D) sublimation point
E) boiling point

A) melting point
B) triple point
C) critical point
D) sublimation point
E) boiling point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
13
Octane has a vapor pressure of 40. torr at 45.1°C and 400. torr at 104.0°C. What is its heat of vaporization?
A) 39.0 kJ/mol
B) 46.0 kJ/mol
C) 590 kJ/mol
D) 710 kJ/mol
E) none of the above
A) 39.0 kJ/mol
B) 46.0 kJ/mol
C) 590 kJ/mol
D) 710 kJ/mol
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
14
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 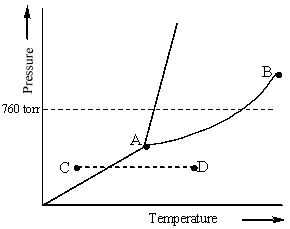
A) Bo(s) has a lower density than Bo(l).
B) The triple point for Bo is at a higher temperature than the melting point for Bo.
C) Bo changes from a solid to a liquid as one follows the line from C to D.
D) Bo changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for Bo.

A) Bo(s) has a lower density than Bo(l).
B) The triple point for Bo is at a higher temperature than the melting point for Bo.
C) Bo changes from a solid to a liquid as one follows the line from C to D.
D) Bo changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for Bo.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
15
The normal boiling point of ether is 307.8 K. Calculate the temperature at which its vapor pressure is exactly half of that at its normal boiling point. The heat of vaporization for ether is 26.69 kJ/mol.
A) 305 K
B) 302 K
C) 295 K
D) 289 K
E) 281 K
A) 305 K
B) 302 K
C) 295 K
D) 289 K
E) 281 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
16
Neon atoms are attracted to each other by
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) intramolecular forces.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) intramolecular forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
17
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 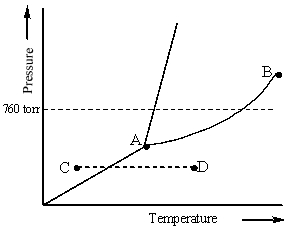
A) increasing temperature with a phase change from solid to liquid
B) increasing temperature with a phase change from solid to vapor
C) increasing temperature with a phase change from liquid to vapor
D) increasing temperature with no phase change
E) increasing temperature beyond the critical point

A) increasing temperature with a phase change from solid to liquid
B) increasing temperature with a phase change from solid to vapor
C) increasing temperature with a phase change from liquid to vapor
D) increasing temperature with no phase change
E) increasing temperature beyond the critical point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
18
Pentane, C5H12, boils at 35°C. Which of the following is true about kinetic energy, Ek, and potential energy, Ep, when liquid pentane at 35°C is compared with pentane vapor at 35°C?
A) Ek(g) < Ek(l); Ep(g) Ep(l)
B) Ek(g) > Ek(l); Ep(g) Ep(l)
C) Ep(g) < Ep(l); Ek(g) Ek(l)
D) Ep(g) > Ep(l); Ek(g) Ek(l)
E) Ep(g) Ep(l); Ek(g) Ek(l)
A) Ek(g) < Ek(l); Ep(g) Ep(l)
B) Ek(g) > Ek(l); Ep(g) Ep(l)
C) Ep(g) < Ep(l); Ek(g) Ek(l)
D) Ep(g) > Ep(l); Ek(g) Ek(l)
E) Ep(g) Ep(l); Ek(g) Ek(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
19
When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
A) polarity
B) polarizability
C) dipole moment
D) van der Waals radius
E) compressibility
A) polarity
B) polarizability
C) dipole moment
D) van der Waals radius
E) compressibility

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
20
A sample of octane in equilibrium with its vapor in a closed 1.0-L container has a vapor pressure of 50.0 torr at 45°C. The container's volume is increased to 2.0 L at constant temperature and the liquid/vapor equilibrium is reestablished. What is the vapor pressure?
A) > 50.0 torr
B) 50.0 torr
C) 25.0 torr
D) The mass of the octane vapor is needed to calculate the vapor pressure.
E) The external pressure is needed to calculate the vapor pressure.
A) > 50.0 torr
B) 50.0 torr
C) 25.0 torr
D) The mass of the octane vapor is needed to calculate the vapor pressure.
E) The external pressure is needed to calculate the vapor pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following atoms should have the smallest polarizability?
A) Si
B) S
C) Te
D) Bi
E) Br
A) Si
B) S
C) Te
D) Bi
E) Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following should have the highest surface tension at a given temperature?
A)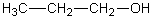
B)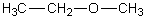
C)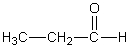
D)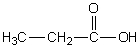
E)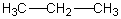
A)
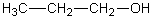
B)
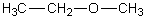
C)
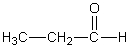
D)
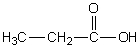
E)
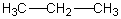

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
23
Comparing the energies of the following intermolecular forces on a kJ/mol basis, which would normally have the highest energy (i.e., be the strongest force)?
A) ion-induced dipole
B) dipole-induced dipole
C) ion-dipole
D) dipole-dipole
E) dispersion
A) ion-induced dipole
B) dipole-induced dipole
C) ion-dipole
D) dipole-dipole
E) dispersion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
24
The strongest intermolecular interactions between pentane (C5H12) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-carbon bonds.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-carbon bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
25
The strongest intermolecular interactions between ethyl alcohol (CH3CH2OH) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-oxygen bonds.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-oxygen bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following has a boiling point which does not fit the general trend?
A) NH3
B) PH3
C) AsH3
D) SbH3
E) BiH3
A) NH3
B) PH3
C) AsH3
D) SbH3
E) BiH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following should have the highest boiling point?
A) CF4
B) CCl4
C) CBr4
D) CI4
E) CH4
A) CF4
B) CCl4
C) CBr4
D) CI4
E) CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
28
The strongest intermolecular interactions between hydrogen sulfide (H2S) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) disulfide linkages.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) disulfide linkages.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
29
What types of forces exist between molecules of CO2?
A) hydrogen bonding only.
B) hydrogen bonding and dispersion forces.
C) dipole-dipole forces only.
D) dipole-dipole and dispersion forces.
E) dispersion forces only.
A) hydrogen bonding only.
B) hydrogen bonding and dispersion forces.
C) dipole-dipole forces only.
D) dipole-dipole and dispersion forces.
E) dispersion forces only.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
30
The strongest intermolecular interactions between hydrogen fluoride (HF) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) ionic bonds.
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) ionic bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which one of the following substances will have hydrogen bonds between molecules?
A) (CH3)3N
B) CH3-O-CH3
C) CH3CH2-OH
D) CH3CH2-F
E) HI
A) (CH3)3N
B) CH3-O-CH3
C) CH3CH2-OH
D) CH3CH2-F
E) HI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
32
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
A)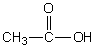 ,
, 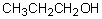
B)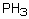 ,
, 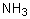
C)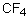 ,
, 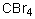
D)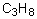 ,
, 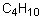
E)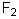 ,
, 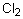
A)
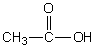 ,
, 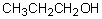
B)
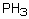 ,
, 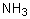
C)
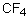 ,
, 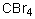
D)
 ,
, 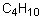
E)
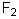 ,
, 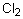

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following should have the highest surface tension at a given temperature?
A) CH4
B) CF4
C) CCl4
D) CBr4
E) CI4
A) CH4
B) CF4
C) CCl4
D) CBr4
E) CI4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following pairs of molecules can form hydrogen bonds between them?
A) HCl and HI
B) CH3OH and NH3
C) CH4 and H2O
D) SO2 and CH2O
E) H2 and O2
A) HCl and HI
B) CH3OH and NH3
C) CH4 and H2O
D) SO2 and CH2O
E) H2 and O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
35
Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first.
A) C7H16, C5H12
B) CCl4, CBr4
C) H2O, H2S
D) CH3CH2OH, CH3-O-CH3
E) Xe, Kr
A) C7H16, C5H12
B) CCl4, CBr4
C) H2O, H2S
D) CH3CH2OH, CH3-O-CH3
E) Xe, Kr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following pairs is arranged with the particle of higher polarizability listed first?
A) Se2-, S2-
B) I, I-
C) Mg2+, Mg
D) Br, I
E) none of the above
A) Se2-, S2-
B) I, I-
C) Mg2+, Mg
D) Br, I
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following pairs is arranged with the particle of higher polarizability listed first?
A) CCl4, CI4
B) H2O, H2Se
C) C6H14, C4H10
D) NH3, NF3
E) none of the above
A) CCl4, CI4
B) H2O, H2Se
C) C6H14, C4H10
D) NH3, NF3
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
38
In which of the following compounds will the molecules not form hydrogen bonds with each other?
A)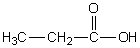
B)
C)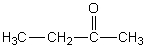
D)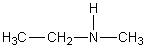
E)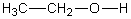
A)
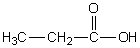
B)

C)
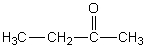
D)
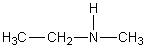
E)
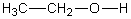

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following should have the lowest boiling point?
A) C5H12
B) C6H14
C) C8H18
D) C10H22
E) C12H26
A) C5H12
B) C6H14
C) C8H18
D) C10H22
E) C12H26

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following atoms should have the greatest polarizability?
A) F
B) Br
C) Po
D) Pb
E) He
A) F
B) Br
C) Po
D) Pb
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
41
A certain solid metallic element has a density 7.87 g/cm3 and a molar mass of 55.85 g/mol. It crystallizes with a cubic unit cell, with an edge length of 286.7 pm. Calculate the number of atoms per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following statements about the packing of monatomic solids with different unit cells is incorrect?
A) The coordination number of atoms in hcp and fcc structures is 12.
B) The coordination number of atoms in simple cubic structures is 6.
C) The coordination number of atoms in bcc structures is 8.
D) A bcc structure has a higher packing efficiency than a simple cubic structure.
E) A bcc structure has a higher packing efficiency than a fcc structure.
A) The coordination number of atoms in hcp and fcc structures is 12.
B) The coordination number of atoms in simple cubic structures is 6.
C) The coordination number of atoms in bcc structures is 8.
D) A bcc structure has a higher packing efficiency than a simple cubic structure.
E) A bcc structure has a higher packing efficiency than a fcc structure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
43
Iron crystallizes in the body-centered cubic lattice. What is the coordination number for Fe?
A) 4
B) 6
C) 8
D) 10
E) 12
A) 4
B) 6
C) 8
D) 10
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
44
A metal such with a face-centered cubic lattice will have ________________ atom(s) per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 10
A) 1
B) 2
C) 3
D) 4
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
45
Crystal structures may be conveniently measured using
A) X-ray diffraction.
B) infrared spectroscopy.
C) ultraviolet-visible spectroscopy.
D) microwave spectroscopy.
E) magnetic resonance imaging.
A) X-ray diffraction.
B) infrared spectroscopy.
C) ultraviolet-visible spectroscopy.
D) microwave spectroscopy.
E) magnetic resonance imaging.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
46
Lead crystallizes in the face-centered cubic lattice. What is the coordination number for Pb?
A) 4
B) 6
C) 8
D) 10
E) 12
A) 4
B) 6
C) 8
D) 10
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
47
When identical particles pack in a simple cubic lattice, there is/are ____ particle(s) per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
48
A cubic unit cell has an edge length of 400. pm. The length of its body diagonal (internal diagonal) in pm is therefore
A) 512.
B) 566.
C) 631.
D) 693.
E) 724.
A) 512.
B) 566.
C) 631.
D) 693.
E) 724.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following factors contributes to a low viscosity for a liquid?
A) low temperature
B) spherical molecular shape
C) hydrogen bonding
D) high molecular weight
E) high boiling point
A) low temperature
B) spherical molecular shape
C) hydrogen bonding
D) high molecular weight
E) high boiling point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following properties measures the energy needed to increase the surface area of a liquid?
A) capillary action
B) surface tension
C) viscosity
D) cohesion
E) specific elasticity
A) capillary action
B) surface tension
C) viscosity
D) cohesion
E) specific elasticity

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following pairs of substances is arranged so that the one with higher viscosity is listed first? 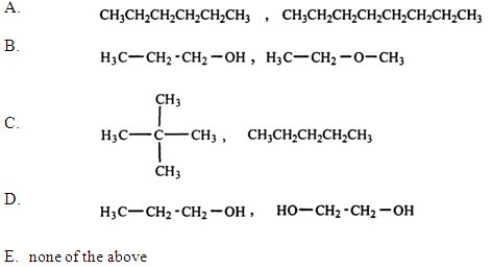
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
52
A metal with a body-centered cubic lattice will have ______ atom(s) per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 9
A) 1
B) 2
C) 3
D) 4
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
53
Polonium crystallizes in the simple cubic lattice. What is the coordination number for Po?
A) 3
B) 4
C) 6
D) 8
E) 12
A) 3
B) 4
C) 6
D) 8
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
54
The meniscus of mercury in a glass capillary tube is convex because of
A) the very high density of mercury as compared with water.
B) the low surface tension of mercury.
C) the greater attraction of mercury atoms to the glass than to each other.
D) the weaker attraction of mercury atoms to the glass than to each other.
E) electrostatic repulsion between the glass and the mercury.
A) the very high density of mercury as compared with water.
B) the low surface tension of mercury.
C) the greater attraction of mercury atoms to the glass than to each other.
D) the weaker attraction of mercury atoms to the glass than to each other.
E) electrostatic repulsion between the glass and the mercury.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which one of the following statements about unit cells and packing in solids is incorrect?
A) In any unit cell of a solid crystal, each face of the cell must have an opposite face which is equal and parallel to it.
B) The faces of a unit cell must all be at angles of 90° to each other.
C) The coordination number of atoms in a close packed metal is 12.
D) The packing efficiency in fcc structures is higher than in bcc structures.
E) The packing efficiency in fcc and hcp structures is the same.
A) In any unit cell of a solid crystal, each face of the cell must have an opposite face which is equal and parallel to it.
B) The faces of a unit cell must all be at angles of 90° to each other.
C) The coordination number of atoms in a close packed metal is 12.
D) The packing efficiency in fcc structures is higher than in bcc structures.
E) The packing efficiency in fcc and hcp structures is the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following terms refers to the resistance of a liquid to flow?
A) surface tension
B) capillary action
C) viscosity
D) adhesion
E) cohesion
A) surface tension
B) capillary action
C) viscosity
D) adhesion
E) cohesion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following statements concerning a face-centered cubic unit cell and the corresponding lattice, made up of identical atoms, is incorrect?
A) The coordination number of the atoms in the lattice is 8.
B) The packing in this lattice is more efficient than for a body-centered cubic system.
C) If the atoms have radius r, then the length of the cube edge is 8 × r.
D) There are four atoms per unit cell in this type of packing.
E) The packing efficiency in this lattice and hexagonal close packing are the same.
A) The coordination number of the atoms in the lattice is 8.
B) The packing in this lattice is more efficient than for a body-centered cubic system.
C) If the atoms have radius r, then the length of the cube edge is 8 × r.
D) There are four atoms per unit cell in this type of packing.
E) The packing efficiency in this lattice and hexagonal close packing are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following liquids is likely to have the highest surface tension?
A) Br2
B) C8H18
C) CH3OCH3
D) CH3OH
E) Pb
A) Br2
B) C8H18
C) CH3OCH3
D) CH3OH
E) Pb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following liquid substances would you expect to have the lowest surface tension?
A) Pb
B) CH3OCH3
C) HOCH2CH2OH
D) H2O
E) CH3CH2OH
A) Pb
B) CH3OCH3
C) HOCH2CH2OH
D) H2O
E) CH3CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
60
When the adhesive forces between a liquid and the walls of a capillary tube are greater than the cohesive forces within the liquid
A) the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a convex meniscus.
B) the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a concave meniscus.
C) the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a convex meniscus.
D) the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a concave meniscus.
E) None of the above will occur.
A) the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a convex meniscus.
B) the liquid level in a capillary tube will rise above the surrounding liquid and the surface in the capillary tube will have a concave meniscus.
C) the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a convex meniscus.
D) the liquid level in a capillary tube will drop below the surrounding liquid and the surface in the capillary tube will have a concave meniscus.
E) None of the above will occur.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
61
When liquid bromine is cooled to form a solid, which of the following types of solid would it form?
A) atomic
B) metallic
C) molecular
D) ionic
E) covalent network
A) atomic
B) metallic
C) molecular
D) ionic
E) covalent network

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
62
Mercury melts at -39°C and boils at 357°C. Draw a diagram of the heating curve of mercury. Label all lines and axes, and clearly indicate the melting and boiling points on your diagram.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
63
a. State the essential requirements for hydrogen bonding to be important in a compound.
b. List four properties of water which are significantly influenced by the presence of hydrogen bonding.
b. List four properties of water which are significantly influenced by the presence of hydrogen bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
64
The vapor pressure of 1-butene is 1.268 atm at 273.15 K and its heat of vaporization is 22.9 kJ/mol. What is the normal boiling point of 1-butene?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
65
When silicon is doped with an element from group 3A(13), the device/material produced is a/an
A) intrinsic semiconductor.
B) p-type semiconductor.
C) n-type semiconductor.
D) p-n junction.
E) transistor.
A) intrinsic semiconductor.
B) p-type semiconductor.
C) n-type semiconductor.
D) p-n junction.
E) transistor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following statements about ceramics is incorrect?
A) Silicon carbide has a diamond-like structure.
B) Boron nitride can exist in both diamond-like and graphite-like forms.
C) Silicon carbide can be prepared by direct reaction of silicon and carbon.
D) Superconducting ceramics present manufacturing difficulties owing to their brittleness.
E) Superconducting ceramic compounds usually incorporate cobalt in a key role.
A) Silicon carbide has a diamond-like structure.
B) Boron nitride can exist in both diamond-like and graphite-like forms.
C) Silicon carbide can be prepared by direct reaction of silicon and carbon.
D) Superconducting ceramics present manufacturing difficulties owing to their brittleness.
E) Superconducting ceramic compounds usually incorporate cobalt in a key role.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
67
What word best describes the type of liquid crystal represented below? 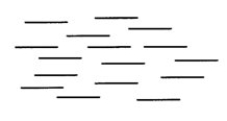
A) nematic
B) cholesteric
C) smectic
D) isotropic
E) elastic

A) nematic
B) cholesteric
C) smectic
D) isotropic
E) elastic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
68
The coordination number of sodium and chloride ions in the NaCl lattice, are, respectively:
A) 10 and 10
B) 8 and 8
C) 6 and 6
D) 4 and 4
E) none of the above
A) 10 and 10
B) 8 and 8
C) 6 and 6
D) 4 and 4
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
69
Consider the phase diagram shown below. 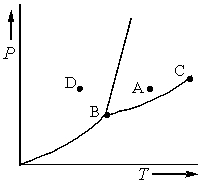 a. What phase(s) is/are present at point A?
a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
 a. What phase(s) is/are present at point A?
a. What phase(s) is/are present at point A?b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
70
The energy gap between the conduction band and the valence band is large for
A) conductors.
B) semiconductors.
C) superconductors.
D) insulators.
E) alloys.
A) conductors.
B) semiconductors.
C) superconductors.
D) insulators.
E) alloys.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
71
The highest temperature at which superconductivity has been achieved is approximately
A) 4 K.
B) 30 K.
C) 70 K.
D) 100 K.
E) 130 K.
A) 4 K.
B) 30 K.
C) 70 K.
D) 100 K.
E) 130 K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
72
For the solid forms of the following elements, which one is most likely to be of the molecular type?
A) Xe
B) C
C) Pb
D) S
E) Cr
A) Xe
B) C
C) Pb
D) S
E) Cr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
73
In an ionic solid MX consisting of the monatomic ions, M+ and X-, the coordination number of M+ is _______________.
A) 1
B) 2
C) 6
D) 8
E) impossible to predict without knowing the crystal structure of MX
A) 1
B) 2
C) 6
D) 8
E) impossible to predict without knowing the crystal structure of MX

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which one of the following substances does not exist in the indicated solid type?
A) graphite - network
B) Na - metallic
C) SiO2 - molecular
D) NaCl - ionic
E) diamond - network
A) graphite - network
B) Na - metallic
C) SiO2 - molecular
D) NaCl - ionic
E) diamond - network

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
75
Chlorine trifluoride is used in processing nuclear reactor fuel. It has a vapor pressure of 29.1 torr at -47.0°C and its heat of vaporization is 30.61 kJ/mol. At what temperature would its vapor pressure be 107.7 torr?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
76
A temperature increase causes __________________ in the conductivity of a conductor.
A) a decrease
B) an increase
C) an increase or decrease (depending on the conductor)
D) a modulation
E) no change
A) a decrease
B) an increase
C) an increase or decrease (depending on the conductor)
D) a modulation
E) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
77
A temperature increase causes __________________ in the conductivity of a semiconductor.
A) a decrease
B) an increase
C) a modulation
D) an increase or decrease (depending on the semiconductor)
E) no change
A) a decrease
B) an increase
C) a modulation
D) an increase or decrease (depending on the semiconductor)
E) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
78
Draw a fully labeled phase diagram (P versus T) of a substance whose solid phase can melt due to applied pressure (i.e., solid is less dense than liquid). Clearly label the triple point and the critical temperature on your diagram.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
79
Liquid ammonia boils at -33.4°C and has a heat of vaporization of 23.5 kJ/mol. Calculate its vapor pressure at -50.0°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck
80
What adjective best describes the solid compound IF7?
A) metallic
B) amorphous
C) covalent network
D) molecular
E) ionic
A) metallic
B) amorphous
C) covalent network
D) molecular
E) ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 111 في هذه المجموعة.
فتح الحزمة
k this deck








