Deck 19: Ionic Equilibria in Aqueous Systems
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/120
العب
ملء الشاشة (f)
Deck 19: Ionic Equilibria in Aqueous Systems
1
A solution is prepared by adding 500 mL of 0.3 M NaClO to 500 mL of 0.4 M HClO. What is the pH of this solution?
A) The pH will be greater than the pKa of hypochlorous acid.
B) The pH will be less than the pKa of hypochlorous acid.
C) The pH will be equal to the pKa of hypochlorous acid.
D) The pH will equal the pKb of sodium hypochlorite.
E) The pH will be none of the above.
A) The pH will be greater than the pKa of hypochlorous acid.
B) The pH will be less than the pKa of hypochlorous acid.
C) The pH will be equal to the pKa of hypochlorous acid.
D) The pH will equal the pKb of sodium hypochlorite.
E) The pH will be none of the above.
The pH will be less than the pKa of hypochlorous acid.
2
Equal volumes of the following pairs of solutions are mixed. Which pair will produce a buffer solution?
A) 0.10 mol L-1 HCl and 0.05 mol L-1 NaOH
B) 0.10 mol L-1 HCl and 0.15 mol L-1 NH3
C) 0.10 mol L-1 HCl and 0.05 mol L-1 NH3
D) 0.10 mol L-1 HCl and 0.20 mol L-1 CH3COOH
E) 0.10 mol L-1 HCl and 0.20 mol L-1 NaCl
A) 0.10 mol L-1 HCl and 0.05 mol L-1 NaOH
B) 0.10 mol L-1 HCl and 0.15 mol L-1 NH3
C) 0.10 mol L-1 HCl and 0.05 mol L-1 NH3
D) 0.10 mol L-1 HCl and 0.20 mol L-1 CH3COOH
E) 0.10 mol L-1 HCl and 0.20 mol L-1 NaCl
0.10 mol L-1 HCl and 0.15 mol L-1 NH3
3
Which one of the following aqueous solutions, when mixed with an equal volume of 0.10 mol L-1 aqueous NH3, will produce a buffer solution?
A) 0.10 mol L-1 HCl
B) 0.20 mol L-1 HCl
C) 0.10 mol L-1 CH3COOH
D) 0.050 mol L-1 NaOH
E) 0.20 mol L-1 NH4Cl
A) 0.10 mol L-1 HCl
B) 0.20 mol L-1 HCl
C) 0.10 mol L-1 CH3COOH
D) 0.050 mol L-1 NaOH
E) 0.20 mol L-1 NH4Cl
0.20 mol L-1 NH4Cl
4
What will be the effect of adding 0.5 mL of 0.1 M HCl to 100 mL of a phosphate buffer in which [H2PO4-] = [HPO42-] = 0.35 M?
A) The pH will increase slightly.
B) The pH will increase significantly.
C) The pH will decrease slightly.
D) The pH will decrease significantly.
E) Since it is a buffer solution, the pH will not be affected.
A) The pH will increase slightly.
B) The pH will increase significantly.
C) The pH will decrease slightly.
D) The pH will decrease significantly.
E) Since it is a buffer solution, the pH will not be affected.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
5
A buffer is to be prepared by adding solid sodium acetate to 0.10 M CH3COOH. Which of the following concentrations of sodium acetate will produce the most effective buffer?
A) 3.0 M CH3COONa
B) 2.5 M CH3COONa
C) 2.0 M CH3COONa
D) 1.5 M CH3COONa
E) 0.30 M CH3COONa
A) 3.0 M CH3COONa
B) 2.5 M CH3COONa
C) 2.0 M CH3COONa
D) 1.5 M CH3COONa
E) 0.30 M CH3COONa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
5
mL of aqueous CH3COOH and 25. mL of aqueous CH3COONa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which, if any, of the following aqueous mixtures would be a buffer system?
A) CH3COOH, NaH2PO4
B) H2CO3, HCO3-
C) H2PO4-, HCO3-
D) HSO4-, HSO3-
E) None of the above will be a buffer solution.
A) CH3COOH, NaH2PO4
B) H2CO3, HCO3-
C) H2PO4-, HCO3-
D) HSO4-, HSO3-
E) None of the above will be a buffer solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
7
A buffer is prepared by adding 0.5 mol of solid sodium hydroxide to 1.0 L of 1.0 M acetic acid (CH3COOH). What is the pH of the buffer?
A) The pH will be pKa - 0.30, where pKa is that of acetic acid.
B) The pH will be greater than the pKa for acetic acid.
C) The pH will be less than the value in answer a.
D) The pH will be equal to the pKa for acetic acid.
E) More information is needed to solve the problem.
A) The pH will be pKa - 0.30, where pKa is that of acetic acid.
B) The pH will be greater than the pKa for acetic acid.
C) The pH will be less than the value in answer a.
D) The pH will be equal to the pKa for acetic acid.
E) More information is needed to solve the problem.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
8
A popular buffer solution consists of carbonate (CO32-) and hydrogen carbonate (HCO3-) conjugate acid-base pair. Which, if any, of the following such buffers has the highest buffer capacity?
A) 0.9 M CO32- and 0.1 M HCO3-
B) 0.1 M CO32- and 0.9 M HCO3-
C) 0.5 M CO32- and 0.5 M HCO3-
D) 0.1 M CO32- and 0.1 M HCO3-
E) They all have the same buffer capacity.
A) 0.9 M CO32- and 0.1 M HCO3-
B) 0.1 M CO32- and 0.9 M HCO3-
C) 0.5 M CO32- and 0.5 M HCO3-
D) 0.1 M CO32- and 0.1 M HCO3-
E) They all have the same buffer capacity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
9
A popular buffer solution consists of carbonate (CO32-) and hydrogen carbonate (HCO3-) conjugate acid-base pair. Which, if any, of the following such buffers can neutralize the greatest amount of added hydrochloric acid, while remaining within its buffer range?
A) 1 L of 0.9 M CO32- and 0.1 M HCO3-
B) 1 L of 0.1 M CO32- and 0.9 M HCO3-
C) 1 L of 0.5 M CO32- and 0.5 M HCO3-
D) 1 L of 0.1 M CO32- and 0.1 M HCO3-
E) They can all neutralize the same amount of hydrochloric acid.
A) 1 L of 0.9 M CO32- and 0.1 M HCO3-
B) 1 L of 0.1 M CO32- and 0.9 M HCO3-
C) 1 L of 0.5 M CO32- and 0.5 M HCO3-
D) 1 L of 0.1 M CO32- and 0.1 M HCO3-
E) They can all neutralize the same amount of hydrochloric acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following has the highest buffer capacity?
A) 0.10 M H2PO4-/0.10 M HPO42-
B) 0.50 M H2PO4-/0.10 M HPO42-
C) 0.10 M H2PO4-/0.50 M HPO42-
D) 0.50 M H2PO4-/0.50 M HPO42-
E) They all have the same buffer capacity.
A) 0.10 M H2PO4-/0.10 M HPO42-
B) 0.50 M H2PO4-/0.10 M HPO42-
C) 0.10 M H2PO4-/0.50 M HPO42-
D) 0.50 M H2PO4-/0.50 M HPO42-
E) They all have the same buffer capacity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
11
A buffer is prepared by adding 100 mL of 0.50 M sodium hydroxide to 100 mL of 0.75 M propanoic acid. Is this a buffer solution, and if so, what is its pH?
A) It is a buffer, pH > pKa of propanoic acid.
B) It is a buffer, pH < pKa of propanoic acid.
C) It is a buffer, pH = pKa of propanoic acid.
D) It is a buffer, pH = pKb of sodium propanoate.
E) Since sodium hydroxide is a strong base, this is not a buffer.
A) It is a buffer, pH > pKa of propanoic acid.
B) It is a buffer, pH < pKa of propanoic acid.
C) It is a buffer, pH = pKa of propanoic acid.
D) It is a buffer, pH = pKb of sodium propanoate.
E) Since sodium hydroxide is a strong base, this is not a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
12
An acetate buffer has a pH of 4.40. Which of the following changes will cause the pH to decrease?
A) dissolving a small amount of solid sodium acetate
B) adding a small amount of dilute hydrochloric acid
C) adding a small amount of dilute sodium hydroxide
D) dissolving a small amount of solid sodium chloride
E) diluting the buffer solution with water
A) dissolving a small amount of solid sodium acetate
B) adding a small amount of dilute hydrochloric acid
C) adding a small amount of dilute sodium hydroxide
D) dissolving a small amount of solid sodium chloride
E) diluting the buffer solution with water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
13
What will be the effect of adding 0.5 mL of 0.1 M NaOH to 100 mL of an acetate buffer in which [CH3COOH] = [CH3COO-] = 0.5 M?
A) The pH will increase slightly.
B) The pH will increase significantly.
C) The pH will decrease slightly.
D) The pH will decrease significantly.
E) Since it is a buffer solution, the pH will not be affected.
A) The pH will increase slightly.
B) The pH will increase significantly.
C) The pH will decrease slightly.
D) The pH will decrease significantly.
E) Since it is a buffer solution, the pH will not be affected.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
14
Buffer solutions with the component concentrations shown below were prepared. Which of them should have the lowest pH?
A) [CH3COOH] = 0.25 M, [CH3COO-] = 0.25 M
B) [CH3COOH] = 0.75 M, [CH3COO-] = 0.75 M
C) [CH3COOH] = 0.75 M, [CH3COO-] = 0.25 M
D) [CH3COOH] = 0.25 M, [CH3COO-] = 0.75 M
E) [CH3COOH] = 1.00 M, [CH3COO-] = 1.00 M
A) [CH3COOH] = 0.25 M, [CH3COO-] = 0.25 M
B) [CH3COOH] = 0.75 M, [CH3COO-] = 0.75 M
C) [CH3COOH] = 0.75 M, [CH3COO-] = 0.25 M
D) [CH3COOH] = 0.25 M, [CH3COO-] = 0.75 M
E) [CH3COOH] = 1.00 M, [CH3COO-] = 1.00 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following aqueous mixtures would be a buffer system?
A) HCl, NaCl
B) HNO3, NaNO3
C) H3PO4, H2PO4-
D) H2SO4, CH3COOH
E) NH3, NaOH
A) HCl, NaCl
B) HNO3, NaNO3
C) H3PO4, H2PO4-
D) H2SO4, CH3COOH
E) NH3, NaOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
16
A solution is prepared by adding 100 mL of 0.2 M hydrochloric acid to 100 mL of 0.4 M sodium formate. Is this a buffer solution, and if so, what is its pH?
A) It is a buffer, pH > pKa of formic acid.
B) It is a buffer, pH < pKa of formic acid.
C) It is a buffer, pH = pKa of formic acid.
D) It is a buffer, pH = pKb of sodium formate.
E) Since hydrochloric acid is a strong acid, this is not a buffer.
A) It is a buffer, pH > pKa of formic acid.
B) It is a buffer, pH < pKa of formic acid.
C) It is a buffer, pH = pKa of formic acid.
D) It is a buffer, pH = pKb of sodium formate.
E) Since hydrochloric acid is a strong acid, this is not a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following acids should be used to prepare a buffer with a pH of 4.5?
A) HOC6H4OCOOH, Ka = 1.0 × 10-3
B) C6H4(COOH)2, Ka = 2.9 × 10-4
C) CH3COOH, Ka = 1.8 × 10-5
D) C5H5O5COOH, Ka = 4.0 × 10-6
E) HBrO, Ka = 2.3 × 10-9
A) HOC6H4OCOOH, Ka = 1.0 × 10-3
B) C6H4(COOH)2, Ka = 2.9 × 10-4
C) CH3COOH, Ka = 1.8 × 10-5
D) C5H5O5COOH, Ka = 4.0 × 10-6
E) HBrO, Ka = 2.3 × 10-9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
18
Citric acid has an acid dissociation constant of 8.4 × 10-4. It would be most effective for preparation of a buffer with a pH of
A) 2.
B) 3.
C) 4.
D) 5.
E) 6.
A) 2.
B) 3.
C) 4.
D) 5.
E) 6.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
19
Buffer solutions with the component concentrations shown below were prepared. Which of them should have the highest pH?
A) [H2PO4-] = 0.50 M, [HPO42-] = 0.50 M
B) [H2PO4-] = 1.0 M, [HPO42-] = 1.0 M
C) [H2PO4-] = 1.0 M, [HPO42-] = 0.50 M
D) [H2PO4-] = 0.50 M, [HPO42-] = 1.0 M
E) [H2PO4-] = 0.75 M, [HPO42-] = 1.0 M
A) [H2PO4-] = 0.50 M, [HPO42-] = 0.50 M
B) [H2PO4-] = 1.0 M, [HPO42-] = 1.0 M
C) [H2PO4-] = 1.0 M, [HPO42-] = 0.50 M
D) [H2PO4-] = 0.50 M, [HPO42-] = 1.0 M
E) [H2PO4-] = 0.75 M, [HPO42-] = 1.0 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
20
A phosphate buffer (H2PO4-/HPO42-) has a pH of 8.3. Which of the following changes will cause the pH to increase?
A) dissolving a small amount of Na2HPO4
B) dissolving a small amount of NaH2PO4
C) adding a small amount of dilute hydrochloric acid
D) adding a small amount of dilute phosphoric acid
E) making the buffer more concentrated by removing some water
A) dissolving a small amount of Na2HPO4
B) dissolving a small amount of NaH2PO4
C) adding a small amount of dilute hydrochloric acid
D) adding a small amount of dilute phosphoric acid
E) making the buffer more concentrated by removing some water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
21
A buffer is prepared by adding 150 mL of 1.0 M NaOH to 250 mL of 1.0 M NaH2PO4. How many moles of HCl must be added to this buffer solution to change the pH by 0.18 units? If necessary, assume the total volume remains unchanged at 400 mL.
A) 0.025 mol HCl
B) 0.063 mol HCl
C) 0.082 mol HCl
D) 0.50 mol HCl
E) 1.0 mol HCl
A) 0.025 mol HCl
B) 0.063 mol HCl
C) 0.082 mol HCl
D) 0.50 mol HCl
E) 1.0 mol HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the [H3O+] in a solution that consists of 0.15 M C2N2H8 (ethylene diamine) and 0.35 C2N2H9Cl? Kb = 4.7 × 10-4
A) 2.0 × 10-3 M
B) 1.1 × 10-3 M
C) 6.3 × 10-9 M
D) 2.1 × 10-10 M
E) 5.0 × 10-11 M
A) 2.0 × 10-3 M
B) 1.1 × 10-3 M
C) 6.3 × 10-9 M
D) 2.1 × 10-10 M
E) 5.0 × 10-11 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the pH of a buffer that consists of 0.45 M CH3COOH and 0.35 M CH3COONa? Ka = 1.8 × 10-5
A) 4.49
B) 4.64
C) 4.85
D) 5.00
E) 5.52
A) 4.49
B) 4.64
C) 4.85
D) 5.00
E) 5.52

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the [H3O+] in a solution that consists of 1.2 M HClO and 2.3 M NaClO? Ka = 3.5 × 10-8
A) 7.8 × 10-9 M
B) 1.8 × 10-8 M
C) 6.7 × 10-8 M
D) 1.6 × 10-7 M
E) none of the above
A) 7.8 × 10-9 M
B) 1.8 × 10-8 M
C) 6.7 × 10-8 M
D) 1.6 × 10-7 M
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the [H3O+] in a buffer that consists of 0.30 M HCOOH and 0.20 M HCOONa? For HCOOH, Ka = 1.7 × 10-4
A) 1.1 × 10-4 M
B) 2.6 × 10-4 M
C) 4.3 × 10-4 M
D) 6.7 × 10-5 M
E) none of the above
A) 1.1 × 10-4 M
B) 2.6 × 10-4 M
C) 4.3 × 10-4 M
D) 6.7 × 10-5 M
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
26
You need to use KH2PO4 and K2HPO4 to prepare a buffer with a pH of 7.45. Which of the following ratios of [base]/[acid] is required? For phosphoric acid, (H3PO4), Ka2 = 6.2 × 10-8.
A) [base]/[acid] = 1.75
B) [base]/[acid] = 1.27
C) [base]/[acid] = 1.24
D) [base]/[acid] = 0.79
E) [base]/[acid] = 0.57
A) [base]/[acid] = 1.75
B) [base]/[acid] = 1.27
C) [base]/[acid] = 1.24
D) [base]/[acid] = 0.79
E) [base]/[acid] = 0.57

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the [H3O+] in a solution that consists of 1.5 M NH3 and 2.5 NH4Cl? Kb = 1.8 × 10-5
A) 1.1 × 10-5 M
B) 3.0 × 10-6 M
C) 3.3 × 10-9 M
D) 9.3 × 10-10 M
E) none of the above
A) 1.1 × 10-5 M
B) 3.0 × 10-6 M
C) 3.3 × 10-9 M
D) 9.3 × 10-10 M
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
28
The pH of blood is 7.35. It is maintained in part by the buffer system composed of carbonic acid (H2CO3) and the bicarbonate (hydrogen carbonate, HCO3-) ion. What is the ratio of [bicarbonate]/[carbonic acid] at this pH? For carbonic acid, Ka1 = 4.2 × 10-7.
A) [bicarbonate]/[carbonic acid] = 0.11
B) [bicarbonate]/[carbonic acid] = 0.38
C) [bicarbonate]/[carbonic acid] = 2.65
D) [bicarbonate]/[carbonic acid] = 9.4
E) None of the above ratios is correct.
A) [bicarbonate]/[carbonic acid] = 0.11
B) [bicarbonate]/[carbonic acid] = 0.38
C) [bicarbonate]/[carbonic acid] = 2.65
D) [bicarbonate]/[carbonic acid] = 9.4
E) None of the above ratios is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
29
Two buffer solutions are prepared using acetic acid and sodium acetate. Solution A contains 20.0 g of acetic acid (CH3COOH) and 5.0 g of sodium acetate CH3COONa). Solution B contains 10.0 g of acetic acid and 25.0 g of sodium acetate. What is the difference between the pH values of these two solutions? For acetic acid, Ka = 1.8 × 10-5.
A) 0.0
B) 1.0
C) 2.0
D) 2.3
E) 4.6
A) 0.0
B) 1.0
C) 2.0
D) 2.3
E) 4.6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the pH of a buffer that consists of 0.20 M NaH2PO4 and 0.40 M Na2HPO4? For NaH2PO4, Ka = 6.2 × 10-8
A) 6.51
B) 6.91
C) 7.51
D) 7.90
E) 8.13
A) 6.51
B) 6.91
C) 7.51
D) 7.90
E) 8.13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
31
A solution is prepared by dissolving 20.0 g of K2HPO4 and 25.0 g of KH2PO4 in enough water to produce 1.0 L of solution. What is the pH of this buffer? For phosphoric acid (H3PO4), Ka2 = 6.2 × 10-8.
A) 7.70
B) 7.42
C) 7.21
D) 7.00
E) 6.72
A) 7.70
B) 7.42
C) 7.21
D) 7.00
E) 6.72

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
32
A formic acid buffer containing 0.50 M HCOOH and 0.50 M HCOONa has a pH of 3.77. What will the pH be after 0.010 mol of NaOH has been added to 100.0 mL of the buffer?
A) 3.67
B) 3.78
C) 3.81
D) 3.85
E) 3.95
A) 3.67
B) 3.78
C) 3.81
D) 3.85
E) 3.95

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
33
A buffer is prepared by adding 300.0 mL of 2.0 M NaOH to 500.0 mL of 2.0 M CH3COOH. What is the pH of this buffer? Ka = 1.8 × 10-5
A) 4.57
B) 4.52
C) 4.87
D) 4.92
E) 4.97
A) 4.57
B) 4.52
C) 4.87
D) 4.92
E) 4.97

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the pKa for the acid HA if a solution of 0.65 M HA and 0.85 M NaA has a pH of 4.75?
A) < 4.00
B) 4.63
C) 4.87
D) 5.02
E) > 5.50
A) < 4.00
B) 4.63
C) 4.87
D) 5.02
E) > 5.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the pH of a solution that consists of 0.50 M H2C6H6O6 (ascorbic acid) and 0.75 M NaHC6H6O6 (sodium ascorbate)? For ascorbic acid, Ka = 6.8 × 10-5
A) 3.76
B) 3.99
C) 4.34
D) 4.57
E) 5.66
A) 3.76
B) 3.99
C) 4.34
D) 4.57
E) 5.66

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
36
You need to prepare a buffer solution with a pH of 4.00, using NaF and HF. What ratio of the ratio of [base]/[acid] should be used in making the buffer? For HF, Ka = 7.2 × 10- 4.
A) [base]/[acid] = 0.14
B) [base]/[acid] = 0.42
C) [base]/[acid] = 2.36
D) [base]/[acid] = 7.20
E) None of the above ratios is correct.
A) [base]/[acid] = 0.14
B) [base]/[acid] = 0.42
C) [base]/[acid] = 2.36
D) [base]/[acid] = 7.20
E) None of the above ratios is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
37
An acetic acid buffer containing 0.50 M CH3COOH and 0.50 M CH3COONa has a pH of 4.74. What will the pH be after 0.0020 mol of HCl has been added to 100.0 mL of the buffer?
A) 4.77
B) 4.71
C) 4.68
D) 4.62
E) none of the above
A) 4.77
B) 4.71
C) 4.68
D) 4.62
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
38
What mass of NaF must be added to 50.0 mL of a 0.500 M HF solution to achieve a pH of 3.25? For HF, Ka = 7.2 × 10-4.
A) 1.3 g
B) 0.69 g
C) 6.9 g
D) 23 g
E) 1.5 g
A) 1.3 g
B) 0.69 g
C) 6.9 g
D) 23 g
E) 1.5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
39
A buffer is prepared by adding 1.00 L of 1.0 M HCl to 750 mL of 1.5 M NaHCOO. What is the pH of this buffer? Ka = 1.7 × 10-4
A) 2.87
B) 3.72
C) 3.82
D) 3.95
E) 4.66
A) 2.87
B) 3.72
C) 3.82
D) 3.95
E) 4.66

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
40
If 10.0 g of NaF and 20.0 g of HF are dissolved in water to make one liter of solution, what will the pH be? For HF, Ka = 6.8 × 10-4.
A) 7.13
B) 2.54
C) 1.57
D) 3.17
E) 4.86
A) 7.13
B) 2.54
C) 1.57
D) 3.17
E) 4.86

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
41
A 25.0-mL sample of 1.00 M NH3 is titrated with 0.15 M HCl. What is the pH of the solution after 15.00 mL of acid have been added to the ammonia solution? Kb = 1.8 × 10-5
A) 10.26
B) 9.30
C) 9.21
D) 8.30
E) 8.21
A) 10.26
B) 9.30
C) 9.21
D) 8.30
E) 8.21

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
42
At the equivalence point in an acid-base titration
A) the [H3O+] equals the Ka of the acid.
B) the [H3O+] equals the Ka of the indicator.
C) the amounts of acid and base which have been combined are in their stoichiometric ratio.
D) the pH is 7.0.
E) the pH has reached a maximum.
A) the [H3O+] equals the Ka of the acid.
B) the [H3O+] equals the Ka of the indicator.
C) the amounts of acid and base which have been combined are in their stoichiometric ratio.
D) the pH is 7.0.
E) the pH has reached a maximum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
43
A 20.0-mL sample of 0.30 M HBr is titrated with 0.15 M NaOH. What is the pH of the solution after 40.3 mL of NaOH have been added to the acid?
A) 2.95
B) 3.13
C) 10.87
D) 11.05
E) 13.14
A) 2.95
B) 3.13
C) 10.87
D) 11.05
E) 13.14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
44
When a strong acid is titrated with a strong base, the pH at the equivalence point
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0, but is not 3.5.
D) is equal to the pKa of the acid.
E) is equal to 3.5.
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0, but is not 3.5.
D) is equal to the pKa of the acid.
E) is equal to 3.5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
45
When a strong acid is titrated with a weak base, the pH at the equivalence point
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is equal to the pKa of the acid.
E) is equal to the pKb of the base.
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is equal to the pKa of the acid.
E) is equal to the pKb of the base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
46
A 20.0-mL sample of 0.25 M HNO3 is titrated with 0.15 M NaOH. What is the pH of the solution after 30.0 mL of NaOH have been added to the acid?
A) 2.00
B) 1.60
C) 1.05
D) 1.00
E) none of the above
A) 2.00
B) 1.60
C) 1.05
D) 1.00
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following indicators would be the best to use when 0.050 M benzoic acid (Ka = 6.6 × 10-5) is titrated with 0.05 M NaOH?
A) bromphenol blue, pH range: 3.0-4.5
B) bromcresol green, pH range: 3.8-5.4
C) alizarin, pH range: 5.7-7.2
D) phenol red, pH range: 6.9-8.2
E) phenolphthalein, pH range: 8.0-10.1
A) bromphenol blue, pH range: 3.0-4.5
B) bromcresol green, pH range: 3.8-5.4
C) alizarin, pH range: 5.7-7.2
D) phenol red, pH range: 6.9-8.2
E) phenolphthalein, pH range: 8.0-10.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
48
When a weak acid is titrated with a weak base, the pH at the equivalence point
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is determined by the sizes of Ka and Kb.
E) is no longer affected by addition of base.
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is determined by the sizes of Ka and Kb.
E) is no longer affected by addition of base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
49
A 25.0-mL sample of 0.35 M HCOOH is titrated with 0.20 M KOH. What is the pH of the solution after 25.0 mL of KOH has been added to the acid? Ka = 1.77 × 10-4
A) 4.00
B) 3.88
C) 3.63
D) 3.51
E) 3.47
A) 4.00
B) 3.88
C) 3.63
D) 3.51
E) 3.47

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
50
A 35.0-mL sample of 0.20 M LiOH is titrated with 0.25 M HCl. What is the pH of the solution after 23.0 mL of HCl have been added to the base?
A) 1.26
B) 1.67
C) 12.33
D) 12.74
E) 13.03
A) 1.26
B) 1.67
C) 12.33
D) 12.74
E) 13.03

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
51
A 10.0-mL sample of 0.75 M CH3CH2COOH is titrated with 0.30 M NaOH. What is the pH of the solution after 22.0 mL of NaOH have been added to the acid? Ka = 1.3 × 10-5
A) 5.75
B) 4.94
C) 4.83
D) 4.02
E) 3.95
A) 5.75
B) 4.94
C) 4.83
D) 4.02
E) 3.95

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
52
The indicator propyl red has Ka = 3.3 × 10-6. What would be the approximate pH range over which it would change color?
A) 3.5-5.5
B) 4.5-6.5
C) 5.5-7.5
D) 6.5-8.5
E) none of the above
A) 3.5-5.5
B) 4.5-6.5
C) 5.5-7.5
D) 6.5-8.5
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
53
When a weak acid is titrated with a strong base, the pH at the equivalence point
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is equal to the pKa of the acid.
E) is equal to 14.0 - pKb , where pKb is that of the base.
A) is greater than 7.0.
B) is equal to 7.0.
C) is less than 7.0.
D) is equal to the pKa of the acid.
E) is equal to 14.0 - pKb , where pKb is that of the base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
54
A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH. The following data were collected during the titration. 
What is the Ka for HClO?
A) 1.1 × 10-7
B) 3.5 × 10-8
C) 1.2 × 10-8
D) 4.9 × 10-11
E) none of the above

What is the Ka for HClO?
A) 1.1 × 10-7
B) 3.5 × 10-8
C) 1.2 × 10-8
D) 4.9 × 10-11
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
55
When 20.0 mL of 0.15 M hydrochloric acid is mixed with 20.0 mL of 0.10 M sodium hydroxide, the pH of the resulting solution is
A) 0.00.
B) 12.40.
C) 1.60.
D) 0.82.
E) 7.00.
A) 0.00.
B) 12.40.
C) 1.60.
D) 0.82.
E) 7.00.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak base (0.10 mol L-1) with HCl of the same concentration?
A)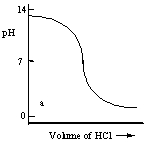
B)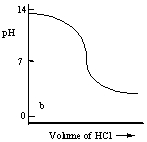
C)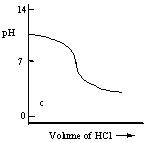
D)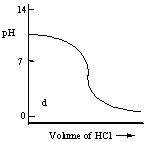
E)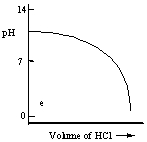
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
57
A 25.0-mL sample of 0.10 M C2H3NH2 (ethylamine) is titrated with 0.15 M HCl. What is the pH of the solution after 9.00 mL of acid have been added to the amine? Kb = 6.5 × 10-4
A) 11.08
B) 10.88
C) 10.74
D) 10.55
E) 10.49
A) 11.08
B) 10.88
C) 10.74
D) 10.55
E) 10.49

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
58
A 50.0-mL sample of 0.50 M HCl is titrated with 0.50 M NaOH. What is the pH of the solution after 28.0 mL of NaOH have been added to the acid?
A) 0.85
B) 0.75
C) 0.66
D) 0.49
E) 3.8
A) 0.85
B) 0.75
C) 0.66
D) 0.49
E) 3.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak acid (0.10 mol L-1) with a strong base of the same concentration?
A)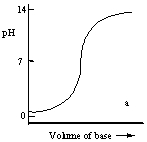
B)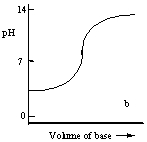
C)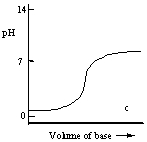
D)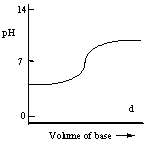
E)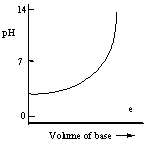
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
60
A diprotic acid H2A has Ka1 = 1 × 10-4 and Ka2 = 1 × 10-8. The corresponding base A2- is titrated with aqueous HCl, both solutions being 0.1 mol L-1. Which one of the following diagrams best represents the titration curve which will be seen?
A)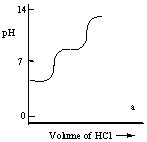
B)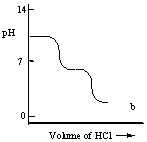
C)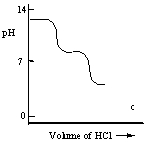
D)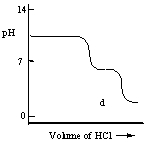
E)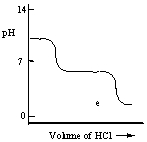
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
61
Calculate the solubility of barium carbonate, BaCO3, in pure water. Ksp = 2.0 × 10-9
A) 1.3 × 10-3 M
B) 3.2 × 10-5 M
C) 2.2 × 10-5 M
D) 4.5 × 10-5 M
E) 4.0 × 10-18 M
A) 1.3 × 10-3 M
B) 3.2 × 10-5 M
C) 2.2 × 10-5 M
D) 4.5 × 10-5 M
E) 4.0 × 10-18 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
62
A saturated solution of calcium hydroxide, Ca(OH)2, is in contact with excess solid Ca(OH)2. Which of the following statements correctly describes what will happen when aqueous HCl (a strong acid) is added to this mixture, and system returns to equilibrium? (For Ca(OH)2, Ksp = 6.5 × 10-6))
A) The solubility of Ca(OH)2 will be unchanged.
B) The OH- concentration will decrease and the Ca2+ concentration will increase.
C) The OH- concentration will increase and the Ca2+ concentration will decrease.
D) The concentrations of both Ca2+ and OH- will increase.
E) The solubility of Ca(OH)2 will decrease.
A) The solubility of Ca(OH)2 will be unchanged.
B) The OH- concentration will decrease and the Ca2+ concentration will increase.
C) The OH- concentration will increase and the Ca2+ concentration will decrease.
D) The concentrations of both Ca2+ and OH- will increase.
E) The solubility of Ca(OH)2 will decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
63
Write the ion product expression for magnesium fluoride, MgF2.
A)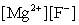
B)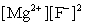
C)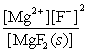
D)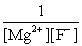
E)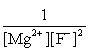
A)
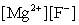
B)
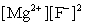
C)
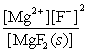
D)
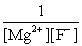
E)
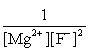

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
64
A change in pH will significantly affect the solubility of which, if any, of the following compounds?
A) BaF2
B) CuCl
C) CuBr
D) AgI
E) None of the solubilities will be significantly affected.
A) BaF2
B) CuCl
C) CuBr
D) AgI
E) None of the solubilities will be significantly affected.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following substances has the greatest solubility in water?
A) MgCO3, Ksp = 3.5 × 10-8
B) NiCO3, Ksp = 1.3 × 10-7
C) AgIO3, Ksp = 3.1 × 10-8
D) CuBr, Ksp = 5.0 × 10-9
E) AgCN, Ksp = 2.2 × 10-16
A) MgCO3, Ksp = 3.5 × 10-8
B) NiCO3, Ksp = 1.3 × 10-7
C) AgIO3, Ksp = 3.1 × 10-8
D) CuBr, Ksp = 5.0 × 10-9
E) AgCN, Ksp = 2.2 × 10-16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
66
Write the ion product expression for silver sulfide, Ag2S.
A)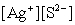
B)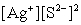
C)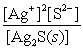
D)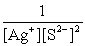
E)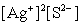
A)
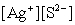
B)
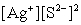
C)
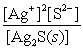
D)
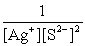
E)
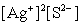

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
67
Calculate the solubility of silver oxalate, Ag2C2O4, in pure water. Ksp = 1.0 × 10-11
A) 1.4 × 10-4 M
B) 8.2 × 10-5 M
C) 5.4 × 10-5 M
D) 3.2 × 10-6 M
E) 2.5 × 10-12 M
A) 1.4 × 10-4 M
B) 8.2 × 10-5 M
C) 5.4 × 10-5 M
D) 3.2 × 10-6 M
E) 2.5 × 10-12 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
68
What volume of 0.200 M KOH must be added to 17.5 mL of 0.135 M H3PO4 to reach the third equivalence point?
A) 3.94 mL
B) 11.8 mL
C) 17.5 mL
D) 23.6 mL
E) 35.4 mL
A) 3.94 mL
B) 11.8 mL
C) 17.5 mL
D) 23.6 mL
E) 35.4 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
69
Calculate the solubility of silver phosphate, Ag3PO4, in pure water. Ksp = 2.6 × 10-18
A) 4.0 × 10-5 M
B) 1.8 × 10-5 M
C) 4.0 × 10-6 M
D) 1.5 × 10-6 M
E) < 1.0 × 10-6 M
A) 4.0 × 10-5 M
B) 1.8 × 10-5 M
C) 4.0 × 10-6 M
D) 1.5 × 10-6 M
E) < 1.0 × 10-6 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
70
The solubility of calcium chromate is 1.56 × 10-3 g/100 mL of solution. What is the Ksp for CaCrO4?
A) 2.4 × 10-4
B) 1.5 × 10-5
C) 7.6 × 10-6
D) 1.0 × 10-8
E) < 1.0 × 10-8
A) 2.4 × 10-4
B) 1.5 × 10-5
C) 7.6 × 10-6
D) 1.0 × 10-8
E) < 1.0 × 10-8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
71
Write the ion product expression for calcium phosphate, Ca3(PO4)2.
A)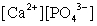
B)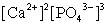
C)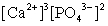
D)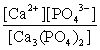
E) None of the above is the correct ion product expression.
A)
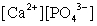
B)
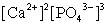
C)
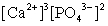
D)
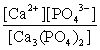
E) None of the above is the correct ion product expression.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
72
The solubility of aluminum hydroxide in water ______________ when dilute nitric acid is added to it.
A) increases
B) decreases
C) does not change
D) first increases, then decreases
E) first decreases, then increases
A) increases
B) decreases
C) does not change
D) first increases, then decreases
E) first decreases, then increases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
73
The solubility of lead(II) chloride is 0.45 g/100 mL of solution. What is the Ksp of PbCl2?
A) 4.9 × 10-2
B) 1.7 × 10-5
C) 8.5 × 10-6
D) 4.2 × 10-6
E) < 1.0 × 10-6
A) 4.9 × 10-2
B) 1.7 × 10-5
C) 8.5 × 10-6
D) 4.2 × 10-6
E) < 1.0 × 10-6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
74
What volume of 0.500 M H2SO4 is needed to react completely with 20.0 mL of 0.400 M LiOH?
A) 4.00 mL
B) 8.00 mL
C) 12.5 mL
D) 16.0 mL
E) 32.0 mL
A) 4.00 mL
B) 8.00 mL
C) 12.5 mL
D) 16.0 mL
E) 32.0 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
75
When 0.300 g of a diprotic acid was titrated with 0.100 M LiOH, 40.0 mL of the LiOH solution was needed to reach the second equivalence point. Identify the formula of the diprotic acid.
A) H2S
B) H2C2O4
C) H2C4H4O6
D) H2Se
E) H2Te
A) H2S
B) H2C2O4
C) H2C4H4O6
D) H2Se
E) H2Te

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following substances has the greatest solubility in water?
A) PbI2, Ksp = 7.9 × 10-9
B) BaF2, Ksp = 1.5 × 10-6
C) Ca(OH)2, Ksp = 6.5 × 10-6
D) Zn(IO3)2, Ksp = 3.9 × 10-6
E) Ag2SO4, Ksp = 1.5 × 10-5
A) PbI2, Ksp = 7.9 × 10-9
B) BaF2, Ksp = 1.5 × 10-6
C) Ca(OH)2, Ksp = 6.5 × 10-6
D) Zn(IO3)2, Ksp = 3.9 × 10-6
E) Ag2SO4, Ksp = 1.5 × 10-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
77
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
A) 37.0 g/mol
B) 81.0 g/mol
C) 128 g/mol
D) 20.3 g/mol
E) 211 g/mol
A) 37.0 g/mol
B) 81.0 g/mol
C) 128 g/mol
D) 20.3 g/mol
E) 211 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
78
The solubility of silver chloride _______________ when dilute nitric is added to it.
A) increases
B) decreases
C) does not change
D) first increases, then decreases
E) first decreases, then increases
A) increases
B) decreases
C) does not change
D) first increases, then decreases
E) first decreases, then increases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck
79
The solubility of magnesium phosphate is 2.27 × 10-3 g/1.0 L of solution. What is the Ksp for Mg3(PO4)2?
A) 6.5 × 10-12
B) 6.0 × 10-14
C) 5.2 × 10-24
D) 4.8 × 10-26
E) 1.0 × 10-26
A) 6.5 × 10-12
B) 6.0 × 10-14
C) 5.2 × 10-24
D) 4.8 × 10-26
E) 1.0 × 10-26

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 120 في هذه المجموعة.
فتح الحزمة
k this deck








