Deck 21: Aldehydes and Ketones Nucleophilic Addition
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/45
العب
ملء الشاشة (f)
Deck 21: Aldehydes and Ketones Nucleophilic Addition
1
Which of the following oxidants would work for the following reaction? 
A) H2Cr2O7
B) FeCl3
C) I2
D) Ag2O

A) H2Cr2O7
B) FeCl3
C) I2
D) Ag2O
H2Cr2O7
2
What is the first step in nucleophilic addition under acidic conditions?
A) Protonation of the nucleophile
B) Addition of the nucleophile
C) Loss of water
D) Protonation of the carbonyl
A) Protonation of the nucleophile
B) Addition of the nucleophile
C) Loss of water
D) Protonation of the carbonyl
Protonation of the carbonyl
3
What is the major organic product obtained from the following sequence of reactions? 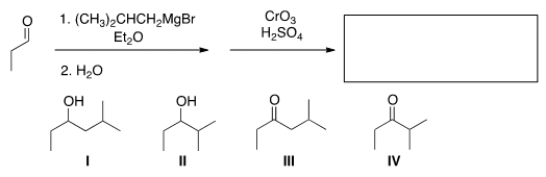
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
III
4
Which is the most reactive carbonyl compound? 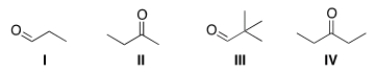
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the driving force for the Wittig reaction?
A) The formation of an alkene
B) The deprotonation of a phosphonium salt
C) The elimination of triphenylphosphine oxide
D) The formation of a phosphonium salt
A) The formation of an alkene
B) The deprotonation of a phosphonium salt
C) The elimination of triphenylphosphine oxide
D) The formation of a phosphonium salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
6
Why are strongly acidic conditions not used in the formation of enamines and imines?
A) The carbonyl will be protonated.
B) The amine will be completely protonated.
C) The product is not stable to strong acid.
D) An enol will be formed.
A) The carbonyl will be protonated.
B) The amine will be completely protonated.
C) The product is not stable to strong acid.
D) An enol will be formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the IUPAC name for the following compound? 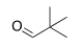
A) pivaldehyde
B) 2,2-dimethylpropanal
C) tert-butyl aldehyde
D) 2,2-dimethylpentanal

A) pivaldehyde
B) 2,2-dimethylpropanal
C) tert-butyl aldehyde
D) 2,2-dimethylpentanal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the structure of 2-trifluoromethyl-2-methoxybutanal? 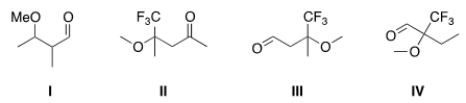
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
9
What would you use to prepare the following ylide from the starting phosphonium salt? 
A) butyl lithium
B) 1-bromo-2-methylpropane
C) triphenylphosphine
D) acetic acid

A) butyl lithium
B) 1-bromo-2-methylpropane
C) triphenylphosphine
D) acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
10
What compound is consistent with the following 1H NMR spectrum? 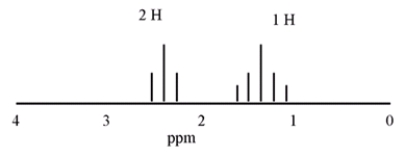
A) Acetone
B) Propanal
C) Cyclobutanone
D) 2-butanone

A) Acetone
B) Propanal
C) Cyclobutanone
D) 2-butanone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the structure of benzophenone? 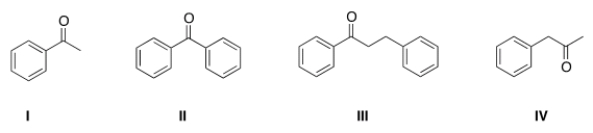
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
12
Is the following reaction reversible and, if so, under what conditions? 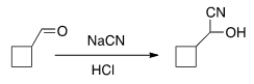
A) No
B) Yes, under acidic conditions
C) Yes, using Pd/C
D) Yes, under basic conditions

A) No
B) Yes, under acidic conditions
C) Yes, using Pd/C
D) Yes, under basic conditions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
13
Why can't you use acidic conditions (such as aqueous hydrochloric acid) for the addition of a Grignard reagent to a ketone?
A) Because the Grignard reagent will react with the acid and be quenched.
B) Because the ketone will be protonated and thus unreactive.
C) Because the ketone will form an unreactive enol.
D) Because the Grignard reagent won't dissolve in aqueous solutions.
A) Because the Grignard reagent will react with the acid and be quenched.
B) Because the ketone will be protonated and thus unreactive.
C) Because the ketone will form an unreactive enol.
D) Because the Grignard reagent won't dissolve in aqueous solutions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the structure of 3-methylcyclohexanone? 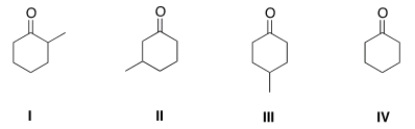
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the product of the following reaction? 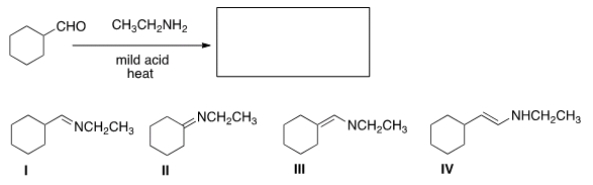
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the product of the following reaction? 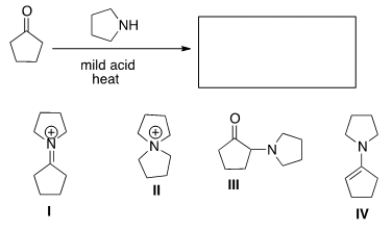
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
17
Using 1H NMR spectroscopy, how can you tell the difference between an aldehyde and a ketone?
A) An aldehyde has a C-H stretch (one or two) between 2700-2830 cm-1.
B) An aldehyde has a proton signal between 9-10 ppm.
C) A ketone has signals around 2-3 ppm.
D) A ketone has a signal around 200 ppm.
A) An aldehyde has a C-H stretch (one or two) between 2700-2830 cm-1.
B) An aldehyde has a proton signal between 9-10 ppm.
C) A ketone has signals around 2-3 ppm.
D) A ketone has a signal around 200 ppm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
18
Using IR spectroscopy, how can you tell the difference between a ketone and an aldehyde?
A) A ketone has no carbonyl stretch at 1720 cm-1.
B) An aldehyde has a carbonyl stretch at 1820 cm-1.
C) An aldehyde has two C-H stretches between 2700-2850 cm-1.
D) A ketone has no C-H stretches.
A) A ketone has no carbonyl stretch at 1720 cm-1.
B) An aldehyde has a carbonyl stretch at 1820 cm-1.
C) An aldehyde has two C-H stretches between 2700-2850 cm-1.
D) A ketone has no C-H stretches.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following will have the highest wave number for the carbonyl stretch in the IR spectrum? 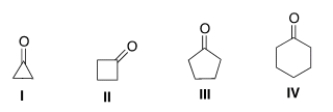
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the product of the following reaction? 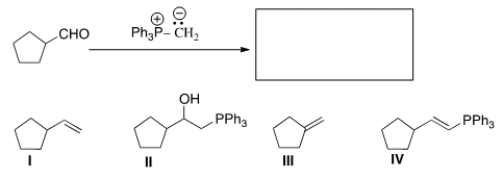
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the product of the following reaction? 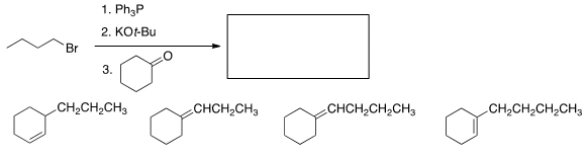
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
22
Name the following aldehyde. 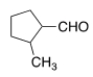
A) 1-methylcyclopentanal
B) 2-methylcyclopentanal
C) 2-methylcyclopentanecarbaldehyde
D) 1-methylcyclopentanylcarbaldehyde

A) 1-methylcyclopentanal
B) 2-methylcyclopentanal
C) 2-methylcyclopentanecarbaldehyde
D) 1-methylcyclopentanylcarbaldehyde

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
23
What sequence of reactions is required for the following transformation? ![<strong>What sequence of reactions is required for the following transformation? </strong> A) [1] NaOMe, [2] acetone B) [1] Ph<sub>3</sub>P, [2] acetone C) [1] Ph<sub>3</sub>P, [2] KOtBu, [3] acetone D) acetone, heat](https://d2lvgg3v3hfg70.cloudfront.net/TB5871/11ea9088_708a_1202_aec7_6142f1afa1fd_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] NaOMe, [2] acetone
B) [1] Ph3P, [2] acetone
C) [1] Ph3P, [2] KOtBu, [3] acetone
D) acetone, heat
![<strong>What sequence of reactions is required for the following transformation? </strong> A) [1] NaOMe, [2] acetone B) [1] Ph<sub>3</sub>P, [2] acetone C) [1] Ph<sub>3</sub>P, [2] KOtBu, [3] acetone D) acetone, heat](https://d2lvgg3v3hfg70.cloudfront.net/TB5871/11ea9088_708a_1202_aec7_6142f1afa1fd_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] NaOMe, [2] acetone
B) [1] Ph3P, [2] acetone
C) [1] Ph3P, [2] KOtBu, [3] acetone
D) acetone, heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the IUPAC for the following compound? 
A) 1-formyl-2-nitropropane
B) 1-formyl-3-nitrobutane
C) 2-nitrobutanal
D) 3-nitrobutanal

A) 1-formyl-2-nitropropane
B) 1-formyl-3-nitrobutane
C) 2-nitrobutanal
D) 3-nitrobutanal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the product of the following reaction? 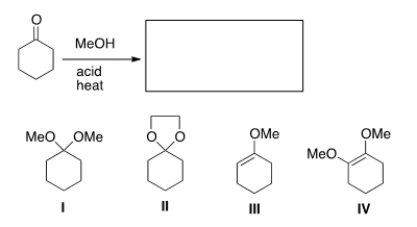
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
26
What is the missing reagent in the reaction below? 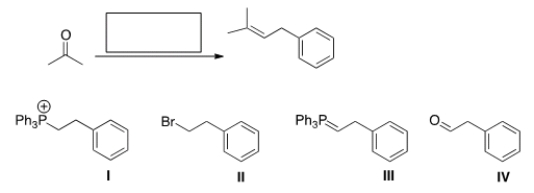
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following products is (are) formed by Wittig reaction of CH3CH2CH2CHO with Ph3P = CHCH3? 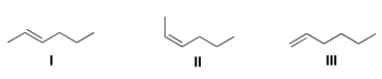
A) Only I
B) Only II
C) Only III
D) Only I and II

A) Only I
B) Only II
C) Only III
D) Only I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
28
What needs to be done to make the following reaction proceed? 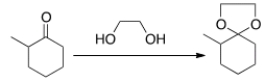
A) Heat the reaction.
B) Add an acid catalyst only.
C) Add a base catalyst only.
D) Heat the reaction and add an acid catalyst.

A) Heat the reaction.
B) Add an acid catalyst only.
C) Add a base catalyst only.
D) Heat the reaction and add an acid catalyst.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
29
What needs to be done to make the following reaction go to starting materials? 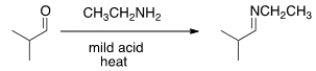
A) Heat the reaction.
B) Add aqueous acid.
C) Add aqueous base.
D) Add water.

A) Heat the reaction.
B) Add aqueous acid.
C) Add aqueous base.
D) Add water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is (are) the product(s) of the following reaction? 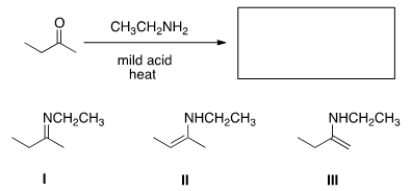
A) I only
B) II only
C) III only
D) II and III

A) I only
B) II only
C) III only
D) II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the product of the following sequence of reactions? 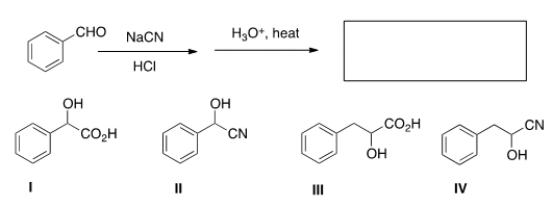
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the missing reagent in the reaction below? 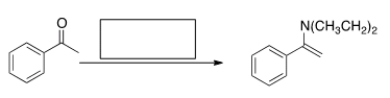
A) Ethyl amine, mild acid
B) Diethylamine, mild acid
C) Diethylamine, strong acid
D) Diethylamine, NaOMe

A) Ethyl amine, mild acid
B) Diethylamine, mild acid
C) Diethylamine, strong acid
D) Diethylamine, NaOMe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the missing reagent in the reaction below? 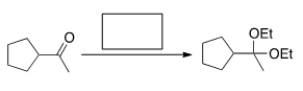
A) Methanol, acid
B) Ethanol, acid
C) NaOEt
D) NaOMe

A) Methanol, acid
B) Ethanol, acid
C) NaOEt
D) NaOMe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the product of the following reaction? 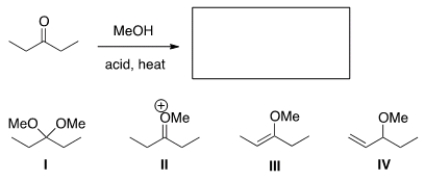
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
35
Butanal (MW= 72) has a boiling point of 76 °C while butanol (MW= 74) has a boiling point of 118 °C. What accounts for this difference in boiling points?
A) Butanol is polar while butanal is not polar.
B) Butanol can exhibit dipole-dipole interactions while butanal cannot.
C) Butanol can hydrogen bond and butanal cannot hydrogen bond.
D) Butanal is less sterically hindered than butanol.
A) Butanol is polar while butanal is not polar.
B) Butanol can exhibit dipole-dipole interactions while butanal cannot.
C) Butanol can hydrogen bond and butanal cannot hydrogen bond.
D) Butanal is less sterically hindered than butanol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
36
How would the following compounds be distinguishable using IR and 1H NMR spectroscopy? 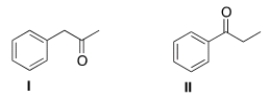
A) The 1H NMR spectrum of compound I will have two singlets.
B) The C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
C) The 1H NMR spectrum of compound II will have one triplet at a chemical shift of about 4.
D) The 1H NMR spectrum of compound I will have two singlets AND the C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.

A) The 1H NMR spectrum of compound I will have two singlets.
B) The C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
C) The 1H NMR spectrum of compound II will have one triplet at a chemical shift of about 4.
D) The 1H NMR spectrum of compound I will have two singlets AND the C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the cyclic hemiacetal product formed from intramolecular cyclization of the following hydroxy aldehyde? 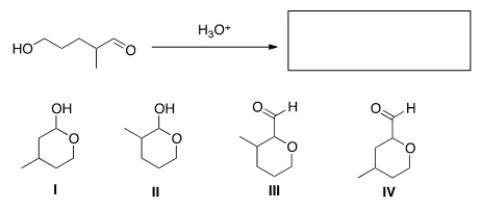
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the product? 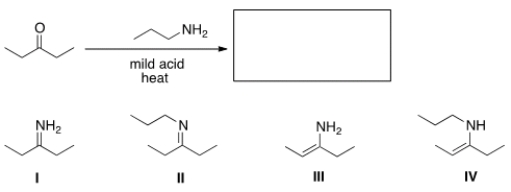
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is (are) the product(s) of the following reaction? 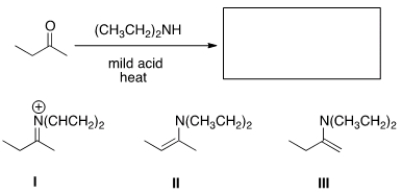
A) I only
B) II only
C) III only
D) II and III

A) I only
B) II only
C) III only
D) II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the product of the following reaction? 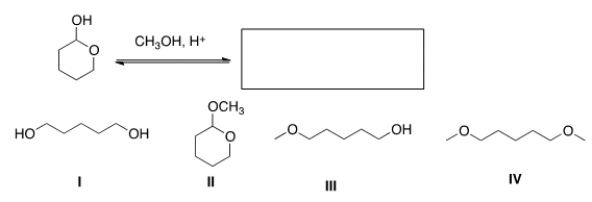
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
41
When an aldehyde is reacted with excess ethanol with an acid as a catalyst, what is the product called?
A) Hemiacetal
B) Di-ether
C) Di-alkoxy alkane
D) Acetal
A) Hemiacetal
B) Di-ether
C) Di-alkoxy alkane
D) Acetal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
42
Cyclic acetals are used as protecting groups for ketones or aldehydes because they are inert to all of the following reagents except
A) aqueous acid.
B) aqueous base.
C) oxidizing reagents.
D) reducing reagents.
A) aqueous acid.
B) aqueous base.
C) oxidizing reagents.
D) reducing reagents.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the product of the following reaction? 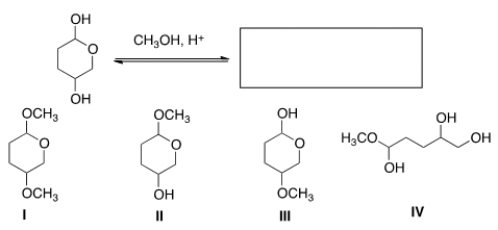
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following statements about carbohydrates is not true?
A) Carbohydrates are sugars and starches.
B) Carbohydrates often contain acetals and hemiacetals.
C) In solution, glucose is in the cyclic acetal form only.
D) Glucose can form a cyclic hemiacetal from the acyclic polyhydroxy aldehyde.
A) Carbohydrates are sugars and starches.
B) Carbohydrates often contain acetals and hemiacetals.
C) In solution, glucose is in the cyclic acetal form only.
D) Glucose can form a cyclic hemiacetal from the acyclic polyhydroxy aldehyde.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
45
Identify how you could synthesize an enamine.
A) React a ketone or an aldehyde with a secondary amine.
B) React a ketone or an aldehyde with a primary amine.
C) React a ylide with a primary amine.
D) React a ylide with a secondary amine.
A) React a ketone or an aldehyde with a secondary amine.
B) React a ketone or an aldehyde with a primary amine.
C) React a ylide with a primary amine.
D) React a ylide with a secondary amine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck








