Deck 13: Mass Spectrometry and Infrared Spectroscopy
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/42
العب
ملء الشاشة (f)
Deck 13: Mass Spectrometry and Infrared Spectroscopy
1
In an IR spectrum, which of the indicated C-H bonds exhibits a stretching absorption at the largest wave number? 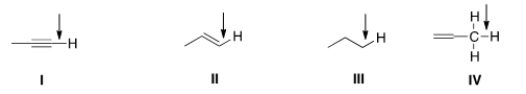
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
I
2
Why is the infrared absorption for the stretching motion of internal alkynes rarely observed?
A) They do not form cations.
B) They are too strong.
C) Stretching in internal alkynes does not involve a change in dipole moment.
D) They do not have hydrogens.
A) They do not form cations.
B) They are too strong.
C) Stretching in internal alkynes does not involve a change in dipole moment.
D) They do not have hydrogens.
Stretching in internal alkynes does not involve a change in dipole moment.
3
Which of the following statements is (are) accurate about the IR spectrum of compounds I, II, and III below? 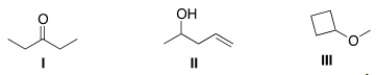
A) Compound I shows absorptions at 2950 and 1700 cm-1.
B) Compound II shows absorptions at 3200-3600 and 1650 cm-1.
C) Compound III shows absorptions at 3200-3600 and 2950 cm-1.
D) Both (Compound I shows absorptions at 2950 and 1700 cm-1) and (Compound II shows absorptions at 3200-3600 and 1650 cm-1) are true.

A) Compound I shows absorptions at 2950 and 1700 cm-1.
B) Compound II shows absorptions at 3200-3600 and 1650 cm-1.
C) Compound III shows absorptions at 3200-3600 and 2950 cm-1.
D) Both (Compound I shows absorptions at 2950 and 1700 cm-1) and (Compound II shows absorptions at 3200-3600 and 1650 cm-1) are true.
Both (Compound I shows absorptions at 2950 and 1700 cm-1) and (Compound II shows absorptions at 3200-3600 and 1650 cm-1) are true.
4
Which of the following statements is (are) true about the IR spectrum of the compound drawn below? 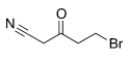
A) It shows absorptions at 3000-3150 cm-1 and 1720 cm-1.
B) It shows absorptions at 3000-2850 cm-1 and 2150 cm-1.
C) It shows absorptions at 2250 cm-1 and 1650 cm-1.
D) It shows absorptions at 2250 cm-1 and 1720 cm-1.
E) Both statements B (It shows absorptions at 3000-2850 cm-1 and 2250 cm-1) and D (It shows absorptions at 2250 cm-1 and 1720 cm-1) are true.

A) It shows absorptions at 3000-3150 cm-1 and 1720 cm-1.
B) It shows absorptions at 3000-2850 cm-1 and 2150 cm-1.
C) It shows absorptions at 2250 cm-1 and 1650 cm-1.
D) It shows absorptions at 2250 cm-1 and 1720 cm-1.
E) Both statements B (It shows absorptions at 3000-2850 cm-1 and 2250 cm-1) and D (It shows absorptions at 2250 cm-1 and 1720 cm-1) are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
5
In electron impact mass spectrometry (EIMS), what is being detected?
A) The molecular mass of the compound
B) The molecular formula of the compound
C) The mass to charge ratio of any ionic species
D) The mass to charge ratio of any neutral species
A) The molecular mass of the compound
B) The molecular formula of the compound
C) The mass to charge ratio of any ionic species
D) The mass to charge ratio of any neutral species

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
6
You are given a bottle of an organic liquid and told it must be either cyclohexane or 1-hexene. Which of the following statements is (are) true about these two compounds? 
A) The two compounds can be differentiated by their mass spectra because they will have molecular ion peaks at different m/z.
B) 1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not.
C) Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1.
D) Statements B (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not) and C (Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1) are both true.
E) Statements A (The two compounds can be differentiated by their mass spectra because they will have molecular ions at different masses), B (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not), and (Both cyclohexane and 1- hexene will show C-H absorptions at about 2950 cm-1) are all true.

A) The two compounds can be differentiated by their mass spectra because they will have molecular ion peaks at different m/z.
B) 1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not.
C) Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1.
D) Statements B (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not) and C (Both cyclohexane and 1-hexene will show C-H absorptions at about 2950 cm-1) are both true.
E) Statements A (The two compounds can be differentiated by their mass spectra because they will have molecular ions at different masses), B (1-Hexene will show an absorption at 1650 cm-1 but cyclohexane will not), and (Both cyclohexane and 1- hexene will show C-H absorptions at about 2950 cm-1) are all true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
7
You observe a compound that exhibits a mass spectrum with peak at 160 and a peak at 162, both of equal intensity. This compound contains:
A) Two chlorine atoms
B) One iodine atom
C) One bromine atom
D) Two bromine atoms
A) Two chlorine atoms
B) One iodine atom
C) One bromine atom
D) Two bromine atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
8
What type of feature will be observed in the EI mass spectrum of chlorobenzene?
A) An absorption at 3100 cm-1
B) A signal at 113.
C) A signal at 112 and a signal at 114 in a ratio of 3:1
D) A signal at 112 and a signal at 114 in a ratio of 1:1
A) An absorption at 3100 cm-1
B) A signal at 113.
C) A signal at 112 and a signal at 114 in a ratio of 3:1
D) A signal at 112 and a signal at 114 in a ratio of 1:1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following statements is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 94, and shows prominent peaks in its IR spectrum at 3600-3200 and 1600 cm-1?
A) The compound has a molecular mass of 94.
B) The compound contains a C=O group and Csp3-H hybridized bonds.
C) The compound contains an OH group and a benzene ring.
D) Both A (The compound has a molecular mass of 94) and B (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
E) Both A (The compound has a molecular mass of 94) and C (The compound contains an OH group and a benzene ring) are true statements.
A) The compound has a molecular mass of 94.
B) The compound contains a C=O group and Csp3-H hybridized bonds.
C) The compound contains an OH group and a benzene ring.
D) Both A (The compound has a molecular mass of 94) and B (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
E) Both A (The compound has a molecular mass of 94) and C (The compound contains an OH group and a benzene ring) are true statements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
10
Consider the three organic compounds drawn below. Which of the following statements is (are) true about the IR spectra of I, II, and III? 
A) I shows strong absorptions at 2950 cm-1 and 1700 cm-1.
B) II shows strong absorptions at 2950 cm-1 and 2250 cm-1.
C) III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1.
D) Statements (I shows strong absorptions at 2950 cm-1 and 1700 cm-1) and (III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1) are true.

A) I shows strong absorptions at 2950 cm-1 and 1700 cm-1.
B) II shows strong absorptions at 2950 cm-1 and 2250 cm-1.
C) III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1.
D) Statements (I shows strong absorptions at 2950 cm-1 and 1700 cm-1) and (III shows strong absorptions at 2950 cm-1 and 3200-3600 cm-1) are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the indicated C-H bonds absorbs at the lowest wave number in the IR spectrum? 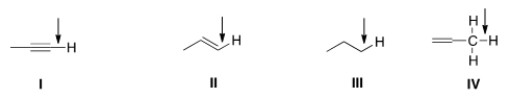
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following statements is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 104, and shows prominent peaks in its IR spectrum at 3200-2850 cm-1?
A) The compound has a molecular mass of 104.
B) The compound contains a C=O group and Csp3-H hybridized bonds.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) Both (The compound has a molecular mass of 104) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
E) Both (The compound has a molecular mass of 104) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
A) The compound has a molecular mass of 104.
B) The compound contains a C=O group and Csp3-H hybridized bonds.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) Both (The compound has a molecular mass of 104) and (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
E) Both (The compound has a molecular mass of 104) and (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
13
A compound X has a molecular ion peak in its mass spectrum at m/z 136. What information does this tell us about X?
A) X has a molecular mass of 136.
B) The molecular formula for X is C8H8O2.
C) The empirical formula for X is C4H4O.
D) Both A (X has a molecular mass of 136) and B (The molecular formula for X is C8H8O2) are true.
E) Statements (X has a molecular mass of 136), (The molecular formula for X is C8H8O2), and (The empirical formula for X is C4H4O) are all true.
A) X has a molecular mass of 136.
B) The molecular formula for X is C8H8O2.
C) The empirical formula for X is C4H4O.
D) Both A (X has a molecular mass of 136) and B (The molecular formula for X is C8H8O2) are true.
E) Statements (X has a molecular mass of 136), (The molecular formula for X is C8H8O2), and (The empirical formula for X is C4H4O) are all true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
14
What type(s) of molecular motion is (are) observed using infrared spectroscopy?
A) Stretching and bending
B) Rotation and excitation
C) Spin flipping
D) Fragmentation
A) Stretching and bending
B) Rotation and excitation
C) Spin flipping
D) Fragmentation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
15
Stronger bonds will be found where in the infrared spectrum?
A) Higher molecular weight
B) Lower molecular weight
C) Lower wavenumbers
D) Higher wavenumbers
A) Higher molecular weight
B) Lower molecular weight
C) Lower wavenumbers
D) Higher wavenumbers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
16
A compound X shows a molecular ion peak at m/z 72 in its mass spectrum, and a strong peak at ~1715 cm-1 in its IR spectrum. Which structures are possible for compound X? 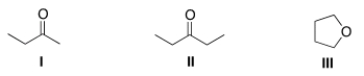
A) I
B) II
C) III
D) I and II

A) I
B) II
C) III
D) I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
17
The functional group region of an infrared spectrum is:
A) Where the cations appear
B) Greater than or equal to 1500 cm-1
C) Less than 1500 cm-1
D) Greater than or equal to 2500 cm-1
A) Where the cations appear
B) Greater than or equal to 1500 cm-1
C) Less than 1500 cm-1
D) Greater than or equal to 2500 cm-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
18
An alkyne C-H bond absorbs at higher wave number than an alkene C-H bond. What does this tell you about the strength of these two bonds?
A) The alkene C-H bond is stronger.
B) The alkyne C-H bond is stronger.
C) The alkene C-C bond is stronger.
D) The alkyne C-C bond is stronger.
A) The alkene C-H bond is stronger.
B) The alkyne C-H bond is stronger.
C) The alkene C-C bond is stronger.
D) The alkyne C-C bond is stronger.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
19
Why does an alkyne carbon-carbon triple bond vibrate at a higher wavenumber than an alkene carbon-carbon double bond?
A) It is stronger.
B) It is weaker.
C) It has fewer hydrogens.
D) It makes a less stable cation.
A) It is stronger.
B) It is weaker.
C) It has fewer hydrogens.
D) It makes a less stable cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
20
Compared to a C-H bond, a C-D bond will vibrate where in the infrared spectrum?
A) Higher molecular weight
B) Lower molecular weight
C) Lower wavenumbers
D) Higher wavenumbers
A) Higher molecular weight
B) Lower molecular weight
C) Lower wavenumbers
D) Higher wavenumbers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
21
Examine the IR below and classify the compound. 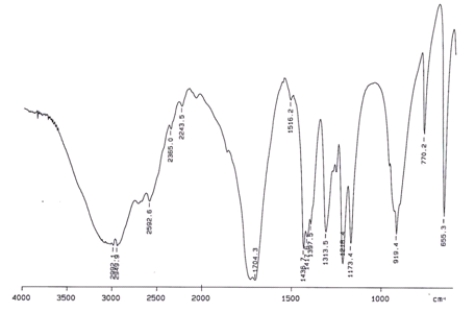
A) Alcohol
B) Aldehyde
C) Carboxylic acid
D) Ketone

A) Alcohol
B) Aldehyde
C) Carboxylic acid
D) Ketone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following statement(s) is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 69, and shows a prominent peak in its IR spectrum at 2250 cm-1?
A) The compound has a molecular mass of 70.
B) The compound contains a C=O group.
C) The compound contains a cyano or alkyne group.
D) Both (The compound has a molecular mass of 70) and (The compound contains a C=O group) are true statements.
E) Both (The compound has a molecular mass of 70) and (The compound contains a cyano or alkyne group) are true statements.
A) The compound has a molecular mass of 70.
B) The compound contains a C=O group.
C) The compound contains a cyano or alkyne group.
D) Both (The compound has a molecular mass of 70) and (The compound contains a C=O group) are true statements.
E) Both (The compound has a molecular mass of 70) and (The compound contains a cyano or alkyne group) are true statements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
23
The base peak in a mass spectrum corresponds to the most stable fragment. Propose a structure for a compound that is consistent with the following data.
(a) The molecular ion peak has m/z = 116
(b) The base peak is at m/z = 59.
(c) The compound is composed of C, H and O atoms.
(d) The IR spectrum shows a strong absorbance at 3257 cm-1.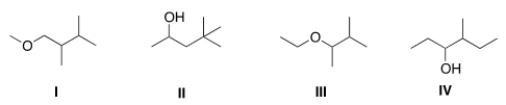
A) I
B) II
C) III
D) IV
(a) The molecular ion peak has m/z = 116
(b) The base peak is at m/z = 59.
(c) The compound is composed of C, H and O atoms.
(d) The IR spectrum shows a strong absorbance at 3257 cm-1.

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
24
Examine the IR below and classify the compound. 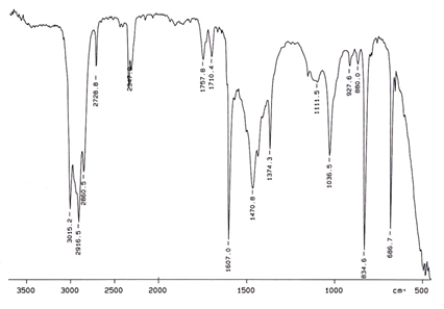
A) Alcohol
B) Arene
C) Amine
D) Ketone
E) Carbocylic acid

A) Alcohol
B) Arene
C) Amine
D) Ketone
E) Carbocylic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
25
What type of signal(s) would you observe in the mass and (or) infrared spectrum of the following compound? 
A) A signal at 1600 cm-1
B) A signal at 3300 cm-1
C) A single mass peak at 92 amu
D) Two mass peaks at 92 and 94 amu

A) A signal at 1600 cm-1
B) A signal at 3300 cm-1
C) A single mass peak at 92 amu
D) Two mass peaks at 92 and 94 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
26
Examine the IR below and classify the compound. 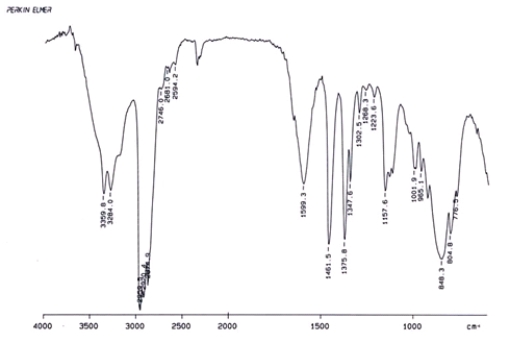
A) Alcohol
B) Aldehyde
C) Amine
D) Ketone

A) Alcohol
B) Aldehyde
C) Amine
D) Ketone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
27
When the phenol shown below is treated with KOH, it forms a product whose IR spectrum does not show an absorption in the 3200-3600 cm-1 region. Propose a structure for the product. 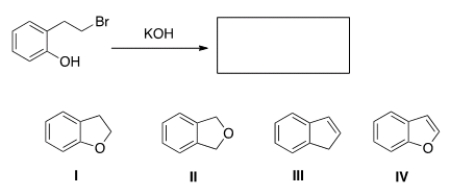
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
28
In a typical mass spectrum, a smaller signal is observed at a mass 1 amu higher than the molecular ion peak. Why?
A) Due to small impurities in the sample
B) Machine error
C) Because a small percentage of the compound will have a carbon that is the isotope 13C instead of 12C
D) Because of fragmentation
A) Due to small impurities in the sample
B) Machine error
C) Because a small percentage of the compound will have a carbon that is the isotope 13C instead of 12C
D) Because of fragmentation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following information is primarily obtained from an IR spectrum?
A) Conjugated system present in a compound.
B) Functional groups present in a compound.
C) Molecular weight of a compound.
D) The carbon and hydrogen framework of a compound.
A) Conjugated system present in a compound.
B) Functional groups present in a compound.
C) Molecular weight of a compound.
D) The carbon and hydrogen framework of a compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
30
An IR spectrum has the following potentially important absorptions: 3091, 3067, 2963, 2921, 2252, 1603, 1499, 1455, 1416, 1078, 1031, 941, 735, and 696 cm-1. Indicate which structure corresponds to the IR data. 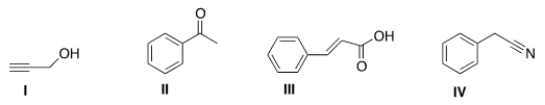
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following compounds will have the lowest wavenumber for carbonyl absorption? 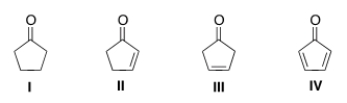
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
32
Examine the IR below and classify the compound. 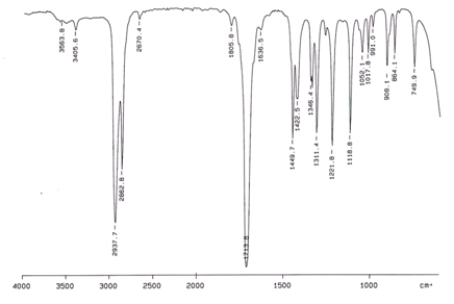
A) Alcohol
B) Aldehyde
C) Amine
D) Ketone
E) Carboxylic acid

A) Alcohol
B) Aldehyde
C) Amine
D) Ketone
E) Carboxylic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
33
What type of signal(s) would you observe in the mass and/or infrared spectrum of the following compound? 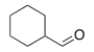
A) A signal at 96 amu
B) Two signals at 112 and 114 amu
C) A signal at 1600 cm-1
D) A signal at 1720 cm-1

A) A signal at 96 amu
B) Two signals at 112 and 114 amu
C) A signal at 1600 cm-1
D) A signal at 1720 cm-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following structures is consistent with a compound that displays a molecular ion peak at 103 and infrared signals at 2250 and 1600 cm-1? 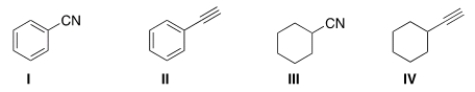
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following statements is (are) true about a compound that has molecular ion peaks in its mass spectrum at mass 170 and 172, and shows prominent peaks in its IR spectrum at 3150-3000 and 1600 cm-1?
A) The compound is not pure.
B) The compound contains a halogen.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) Both A (The compound is not pure) and B (The compound contains a halogen) are true statements.
E) Both A (The compound is not pure) and C (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.
A) The compound is not pure.
B) The compound contains a halogen.
C) The compound contains an OH group and Csp3-H hybridized bonds.
D) Both A (The compound is not pure) and B (The compound contains a halogen) are true statements.
E) Both A (The compound is not pure) and C (The compound contains an OH group and Csp3-H hybridized bonds) are true statements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
36
13C NMR is a technique in which the total number of signals represents the number of unique carbon atoms in a molecule. Propose a structure that is consistent with the following data.
(a) The IR includes peaks at 1603 and 1495 cm-1.
(b) The 13C NMR has a total of 7 signals.
(c) The compound has one acidic proton.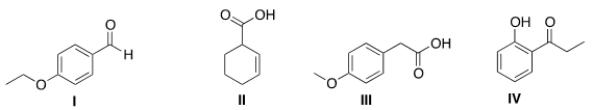
A) I
B) II
C) III
D) IV
(a) The IR includes peaks at 1603 and 1495 cm-1.
(b) The 13C NMR has a total of 7 signals.
(c) The compound has one acidic proton.

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following structures is consistent with a compound that displays a molecular ion peak at 56 and infrared signals at 2250 and 3600-3200 cm-1? 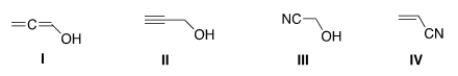
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following structures is consistent with a compound that displays a molecular ion peak at 84 and infrared signals at 3000-2850 cm-1 and no signals between 3000-3300 cm-1? 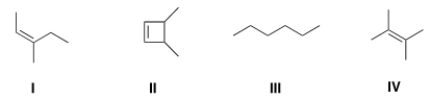
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
39
What type of signal(s) would you observe in the mass and (or) infrared spectrum of the following compound? 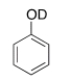
A) A signal at 95 amu
B) A signal at 94 amu
C) Two signals at 95 and 94 amu
D) A signal at 3600-3200 cm-1

A) A signal at 95 amu
B) A signal at 94 amu
C) Two signals at 95 and 94 amu
D) A signal at 3600-3200 cm-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
40
Examine the IR below and classify the compound. 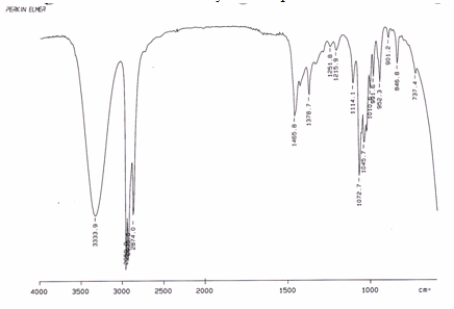
A) Alkane
B) Carboxylic acid
C) Alcohol
D) Alkene

A) Alkane
B) Carboxylic acid
C) Alcohol
D) Alkene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following bonds is IR inactive? 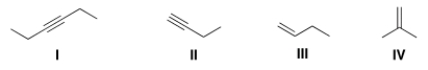
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following statements about the base peak of a mass spectrum is always true?
A) The base peak corresponds to the molecular ion.
B) The base peak corresponds to the most abundant ion.
C) The base peak corresponds to the lowest m/z.
D) None of the above.
A) The base peak corresponds to the molecular ion.
B) The base peak corresponds to the most abundant ion.
C) The base peak corresponds to the lowest m/z.
D) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 42 في هذه المجموعة.
فتح الحزمة
k this deck








