Deck 10: Radical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/114
العب
ملء الشاشة (f)
Deck 10: Radical Reactions
1
For which of the following reactions would the transition state most resemble the products? The following bond dissociation energies may be useful. (CH3)2CH-H CH3CH2CH2-H H-F
(413 kJ mol-1) (423 kJ mol-1) (570 kJ mol-1)
H-Cl H-Br
(432 kJ mol-1) (366 kJ mol-1)
A)

B) CH3CH2CH3 + F· CH3CH2CH2· + HF
C)

D) CH3CH2CH3 + Cl· CH3CH2CH2· + HCl
E) CH3CH2CH3 + Br· CH3CH2CH2· + HBr
(413 kJ mol-1) (423 kJ mol-1) (570 kJ mol-1)
H-Cl H-Br
(432 kJ mol-1) (366 kJ mol-1)
A)

B) CH3CH2CH3 + F· CH3CH2CH2· + HF
C)

D) CH3CH2CH3 + Cl· CH3CH2CH2· + HCl
E) CH3CH2CH3 + Br· CH3CH2CH2· + HBr
CH3CH2CH3 + Br· CH3CH2CH2· + HBr
2
Which of the reactions listed below would be exothermic?
A) CH3-CH3 2CH3·
B) CH3· + CH4 CH4 + CH3·
C) 2(CH3)2CH· (CH3)2CH-CH(CH3)2
D) H· + (CH3)3CH (CH3)3CH + H·
E) None of the above
A) CH3-CH3 2CH3·
B) CH3· + CH4 CH4 + CH3·
C) 2(CH3)2CH· (CH3)2CH-CH(CH3)2
D) H· + (CH3)3CH (CH3)3CH + H·
E) None of the above
2(CH3)2CH· (CH3)2CH-CH(CH3)2
3
How many different monochlorobutanes (including stereoisomers)are formed in the free radical chlorination of butane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
3
4
A chain reaction is one that:
A) involves a series of steps.
B) involves two steps of equal activation energy.
C) is one that can be initiated by light.
D) involves a series of steps,each of which generates a reactive intermediate that brings about the next step.
E) involves free radicals that have an unusual stability and thereby cause a large quantum yield.
A) involves a series of steps.
B) involves two steps of equal activation energy.
C) is one that can be initiated by light.
D) involves a series of steps,each of which generates a reactive intermediate that brings about the next step.
E) involves free radicals that have an unusual stability and thereby cause a large quantum yield.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the reactions listed below would have a value of H° equal to zero?
A) H-H 2H·
B) H· + CH3-H CH3-H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) Reactions (B)and (D)
A) H-H 2H·
B) H· + CH3-H CH3-H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) Reactions (B)and (D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
6
The bond dissociation energies for the relevant bonds are given below each of the species involved in the following reaction.Calculate the overall H° for the reaction. 
A) +437 kJ / mol
B) -437 kJ / mol
C) -411 kJ / mol
D) +26 kJ / mol
E) -1581 kJ / mol

A) +437 kJ / mol
B) -437 kJ / mol
C) -411 kJ / mol
D) +26 kJ / mol
E) -1581 kJ / mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following free radicals is the most stable?
A)
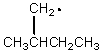
B)
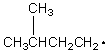
C)
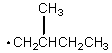
D)
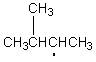
E)
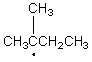
A)
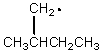
B)
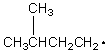
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of these molecules is not expected to arise as a product of the high temperature chlorination of methane?
A) CCl4
B) HCCl3
C) CH2Cl2
D) CH3CH3
E) CH2=CH2
A) CCl4
B) HCCl3
C) CH2Cl2
D) CH3CH3
E) CH2=CH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
9
The reaction of Cl2 with a methyl radical has a positive H°.Which of these drawings is the best representation of the transition state of this reaction?
A)
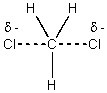
B)
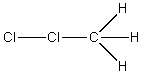
C)
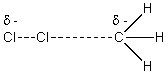
D)
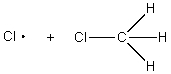
E)
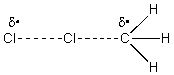
A)

B)

C)
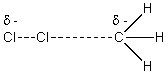
D)
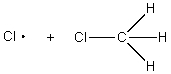
E)
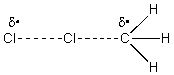

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
10
The bond dissociation energies for the relevant bonds are given below each of the species involved in the following reaction.Calculate the overall H° for the reaction. 
A) +243 kJ / mol
B) -138 kJ / mol
C) +138 kJ / mol
D) -781 kJ / mol
E) +781 kJ / mol

A) +243 kJ / mol
B) -138 kJ / mol
C) +138 kJ / mol
D) -781 kJ / mol
E) +781 kJ / mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
11
If chlorocyclopentane were chlorinated to form all possible dichloro compounds and the product mixture subjected to precise fractional distillation,how many fractions would be obtained (ideally)?
A) 3
B) 4
C) 5
D) 7
E) 9
A) 3
B) 4
C) 5
D) 7
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
12
The bond dissociation energies for the relevant bonds are given below each of the species involved in the following reaction.Calculate the overall H° for the reaction. 
A) -121 kJ / mol
B) +121 kJ / mol
C) +243 kJ / mol
D) +664 kJ / mol
E) -785 kJ / mol

A) -121 kJ / mol
B) +121 kJ / mol
C) +243 kJ / mol
D) +664 kJ / mol
E) -785 kJ / mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
13
The bond dissociation energies for the relevant bonds are given below each of the species involved in the following reaction.Calculate the overall H° for the reaction. 
A) +616 kJ / mol
B) -101 kJ / mol
C) -173 kJ / mol
D) +57 kJ kJ / mol
E) -44 kJ / mol

A) +616 kJ / mol
B) -101 kJ / mol
C) -173 kJ / mol
D) +57 kJ kJ / mol
E) -44 kJ / mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following gas-phase reactions is a possible chain-terminating step in the light-initiated chlorination of methane?
A) Cl-Cl 2Cl·
B) Cl· + CH4 CH3· + H-Cl
C) CH3· + CH3· CH3-CH3
D) CH3· + Cl-Cl CH3Cl + Cl·
E) More than one of the above
A) Cl-Cl 2Cl·
B) Cl· + CH4 CH3· + H-Cl
C) CH3· + CH3· CH3-CH3
D) CH3· + Cl-Cl CH3Cl + Cl·
E) More than one of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the reactions listed below would be exothermic?
A) H-H 2H·
B) H· + CH3-H CH3-H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) All of the above
A) H-H 2H·
B) H· + CH3-H CH3-H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
16
The reaction of 2-methylbutane with chlorine would yield how many monochloro derivatives? (include stereoisomers)
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
17
In the presence of light,ethane (1 mol)reacts with chlorine (1 mol)to form which product(s)?
A) CH2ClCHCl2
B) CH3CHCl2
C) CH3CH2Cl
D) ClCH2CH2Cl
E) All of these
A) CH2ClCHCl2
B) CH3CHCl2
C) CH3CH2Cl
D) ClCH2CH2Cl
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
18
The H° value is expected to be least for which indicated C-H bond of isopentane?
A)
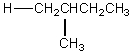
B)
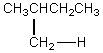
C)
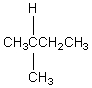
D)
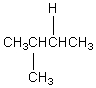
E)
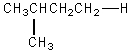
A)

B)
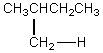
C)

D)

E)
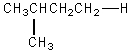

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
19
Hydrogen atom abstraction from which position would yield the most stable free radical intermediate during the reaction of bromine with 2,2,3-trimethylpentane?
A) C1
B) C2
C) C3
D) C4
E) C5
A) C1
B) C2
C) C3
D) C4
E) C5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
20
An alternate mechanism for the chlorination of methane is: Cl2 2Cl·
Cl-Cl, H° = 243 kJ mol-1
Cl· + CH4 CH3Cl + H·
CH3-H, H° = 440 kJ mol-1
H· + Cl2 HCl + Cl·
CH3-Cl, H° = 352 kJ mol-1
H-Cl, H° = 432 kJ mol -1
This mechanism is unlikely because:
A) The overall H° is highly endothermic.
B) The probability factor is low.
C) One of the chain propagating steps is non-productive.
D) One of the chain propagating steps has a very high Eact.
E) One of the chain propagating steps is highly exothermic.
Cl-Cl, H° = 243 kJ mol-1
Cl· + CH4 CH3Cl + H·
CH3-H, H° = 440 kJ mol-1
H· + Cl2 HCl + Cl·
CH3-Cl, H° = 352 kJ mol-1
H-Cl, H° = 432 kJ mol -1
This mechanism is unlikely because:
A) The overall H° is highly endothermic.
B) The probability factor is low.
C) One of the chain propagating steps is non-productive.
D) One of the chain propagating steps has a very high Eact.
E) One of the chain propagating steps is highly exothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
21
In the presence of light at 25°C,isobutane (1 mol)and bromine (1 mol)yield which monobromo product(s)?
A) 2-Methyl-1-bromopropane (almost exclusively)
B) 2-Methyl-2-bromopropane (almost exclusively)
C) A mixture of 50% (A)and 50% (B)
D) A mixture of 90% (A)and 10% (B)
E) Butyl bromide
A) 2-Methyl-1-bromopropane (almost exclusively)
B) 2-Methyl-2-bromopropane (almost exclusively)
C) A mixture of 50% (A)and 50% (B)
D) A mixture of 90% (A)and 10% (B)
E) Butyl bromide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following reactions would have an activation energy equal to zero?
A) H-H 2H·
B) H· + CH3-H CH3--H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) All of the above
A) H-H 2H·
B) H· + CH3-H CH3--H + H·
C) CH3· + CH3· CH3-CH3
D) CH3· + CH3-H CH3-H + CH3·
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following reactions would have the smallest energy of activation?
A)
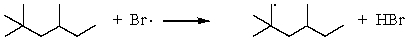
B)

C)
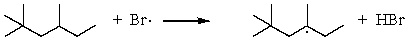
D)
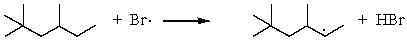
E)
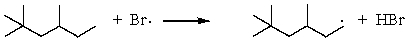
A)
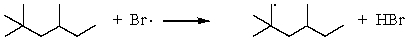
B)

C)
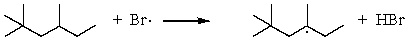
D)
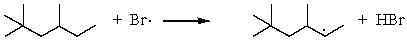
E)
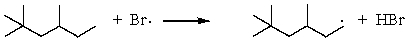

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following statements is true when used to compare the reaction of fluorine with 2-methylhexane and the reaction of bromine with 2-methylhexane?
A) Bromine is the less reactive and the less selective,giving 2-bromo-2-methyl hexane as one of several products.
B) Fluorine is the less reactive and the more selective,giving 2-fluoro-2-methyl hexane as the major product.
C) Fluorine is the more reactive and less selective,giving 2-fluoro-2-methyl hexane as one of several products.
D) Bromine is the more reactive and the more selective,giving 2-bromo-2-methyl hexane as the major product.
E) More than one of the above statements is true.
A) Bromine is the less reactive and the less selective,giving 2-bromo-2-methyl hexane as one of several products.
B) Fluorine is the less reactive and the more selective,giving 2-fluoro-2-methyl hexane as the major product.
C) Fluorine is the more reactive and less selective,giving 2-fluoro-2-methyl hexane as one of several products.
D) Bromine is the more reactive and the more selective,giving 2-bromo-2-methyl hexane as the major product.
E) More than one of the above statements is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
25
For which of the following gas-phase reactions would the Eact be equal to H°?
A) Cl-Cl 2Cl·
B) 2 Cl· Cl-Cl
C) Cl· + CH4 CH3· + H-Cl
D) CH3· + CH3· CH3-CH3
E) CH3· + Cl-Cl CH3-Cl + Cl·
A) Cl-Cl 2Cl·
B) 2 Cl· Cl-Cl
C) Cl· + CH4 CH3· + H-Cl
D) CH3· + CH3· CH3-CH3
E) CH3· + Cl-Cl CH3-Cl + Cl·

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following reactions would have an activation energy equal to zero?
A) CH3-CH3 -- 2CH3·
B) H· + CH3CH3 -- CH3CH3 + H·
C) 2CH3CH2· -- CH3CH2CH2CH3
D) CH3· + CH3CH3 -- CH3CH3 + CH3·
E) None of the above
A) CH3-CH3 -- 2CH3·
B) H· + CH3CH3 -- CH3CH3 + H·
C) 2CH3CH2· -- CH3CH2CH2CH3
D) CH3· + CH3CH3 -- CH3CH3 + CH3·
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
27
Select the structure of the major product formed in the following reaction. 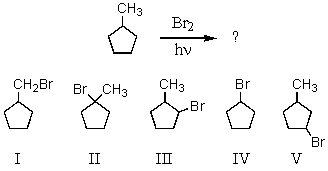
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
28
What feature would you expect to see in the 1H NMR spectrum of B after subjecting A to the following reaction? 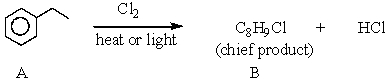
A) There would be only 4 aromatic protons at low field.
B) The signal for the protons on the benzylic carbon would be a doublet.
C) The signal for the methyl protons would be a triplet.
D) The signal for the methyl protons would be a doublet.
E) The signal for the methyl protons would integrate for only 2 hydrogens.

A) There would be only 4 aromatic protons at low field.
B) The signal for the protons on the benzylic carbon would be a doublet.
C) The signal for the methyl protons would be a triplet.
D) The signal for the methyl protons would be a doublet.
E) The signal for the methyl protons would integrate for only 2 hydrogens.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
29
At some temperatures,the relative reactivities of 3°,2° and 1° alkane hydrogens in free radical chlorination are in the ratio of 5:3:1.Thus,monochlorination of isopentane should produce these percentages of 2-chloro-2-methylbutane (A),combined 1-chloro-2-methylbutane and 1-chloro-3-methylbutane (B),and 2-chloro-3-methylbutane (C):
A) 8% A,75% B,17% C
B) 25% A,45% B,30% C
C) 29% A,44% B,18% C
D) 30% A,35% B,35% C
E) 36% A,43% B,21% C
A) 8% A,75% B,17% C
B) 25% A,45% B,30% C
C) 29% A,44% B,18% C
D) 30% A,35% B,35% C
E) 36% A,43% B,21% C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which reaction would you expect to have the smallest energy of activation? H°(kJ mol-1)
A) CH3· + CH3· -- CH3CH3 -378
B) CH4 + F· -- CH3· + HF -130
C) CH4 + I· -- CH3· + HI +142
D) CH4 + Br· -- CH3· + HBr +104
E) CH4 + Cl· -- CH3· + HCl +8
A) CH3· + CH3· -- CH3CH3 -378
B) CH4 + F· -- CH3· + HF -130
C) CH4 + I· -- CH3· + HI +142
D) CH4 + Br· -- CH3· + HBr +104
E) CH4 + Cl· -- CH3· + HCl +8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
31
An example of a reaction having an Eact = 0 would be:
A) Br· + Br-Br -- Br-Br + Br·
B) F· + CH4 -- H-F + CH3·
C) CH3· + Cl· -- CH3Cl
D) More than one of these
E) None of these
A) Br· + Br-Br -- Br-Br + Br·
B) F· + CH4 -- H-F + CH3·
C) CH3· + Cl· -- CH3Cl
D) More than one of these
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
32
What sequence of reactions could be used to prepare cis-1,2-cyclopentanediol from cyclopentane?
A) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)OsO4; (4)NaHSO3/H2O
B) (1)t-BuOK/t-BuOH; (2)Cl2,h ; (3)NaOH/H2O
C) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)H2O2
D) (1)NaOH/H2O; (2)Br2; (3)NaNH2(2eq. )/liq.NH3; (4)KMnO4,NaOH/H2O,5°C
E) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)Hg(OAc)2,H2O (4)NaBH4,H3O+
A) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)OsO4; (4)NaHSO3/H2O
B) (1)t-BuOK/t-BuOH; (2)Cl2,h ; (3)NaOH/H2O
C) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)H2O2
D) (1)NaOH/H2O; (2)Br2; (3)NaNH2(2eq. )/liq.NH3; (4)KMnO4,NaOH/H2O,5°C
E) (1)Cl2,h ; (2)t-BuOK/t-BuOH; (3)Hg(OAc)2,H2O (4)NaBH4,H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following reactions would have the smallest energy of activation?
A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C)
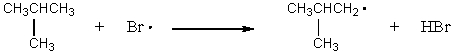
D)
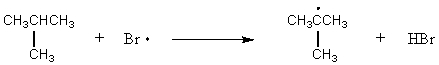
E)
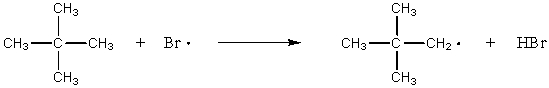
A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
34
An example of a reaction having an Eact = 0 would be:
A) Br· + Br-Br Br-Br + Br·
B) F· + CH4 H-F + CH3·
C) CH3· + CH3CH3 CH4 + CH3CH2·
D) Br· + H-Br H-Br + Br·
E) CH3· + CH3· CH3-CH3
A) Br· + Br-Br Br-Br + Br·
B) F· + CH4 H-F + CH3·
C) CH3· + CH3CH3 CH4 + CH3CH2·
D) Br· + H-Br H-Br + Br·
E) CH3· + CH3· CH3-CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which reaction would you expect to have the largest energy of activation? H° (kJ mol-1)
A) CH3· + CH3· CH3CH3 -378
B) CH3· + Br· CH3Br -130
C) CH4 + I· CH3· + HI +142
D) CH4 + Br· CH3· + HBr +104
E) CH4 + Cl· CH3· + HCl +8
A) CH3· + CH3· CH3CH3 -378
B) CH3· + Br· CH3Br -130
C) CH4 + I· CH3· + HI +142
D) CH4 + Br· CH3· + HBr +104
E) CH4 + Cl· CH3· + HCl +8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
36
Mono-bromination of the following alkane, 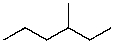 , (using Br2 with light)would be?
, (using Br2 with light)would be? 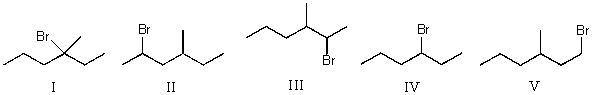
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following reactions should have the smallest energy of activation?
A) CH4 + Cl· CH3· + HCl
B) CH3CH3 + Cl· CH3CH2· + HCl
C)
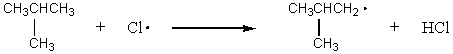
D)
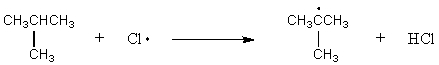
E)
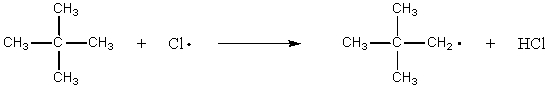
A) CH4 + Cl· CH3· + HCl
B) CH3CH3 + Cl· CH3CH2· + HCl
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
38
When an alkane in which all hydrogen atoms are not equivalent is monosubstituted,use of this halogen produces a ratio of isomers which is essentially statistical,i.e. ,dependent only on the number of each type of hydrogen.
A) F2
B) Cl2
C) Br2
D) I2
E) All of the above
A) F2
B) Cl2
C) Br2
D) I2
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
39
Mono-bromination of the following alkane, 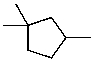 , (using Br2 with light)would be?
, (using Br2 with light)would be? 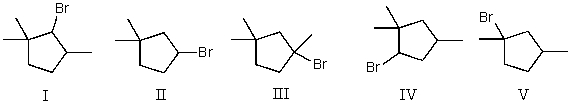
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which is true for a chain-terminating step?
A) "A new free radical is formed."
B) "The process is endothermic."
C) "Eact = 0."
D) " H° is positive."
E) "A product is formed which is immune to further reaction."
A) "A new free radical is formed."
B) "The process is endothermic."
C) "Eact = 0."
D) " H° is positive."
E) "A product is formed which is immune to further reaction."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
41
For which reaction would the transition state be most product-like?
A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C)
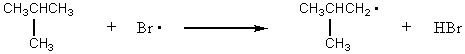
D)
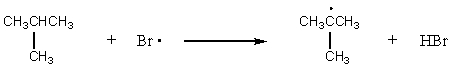
E)

A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the product for the following three-step reaction sequence? 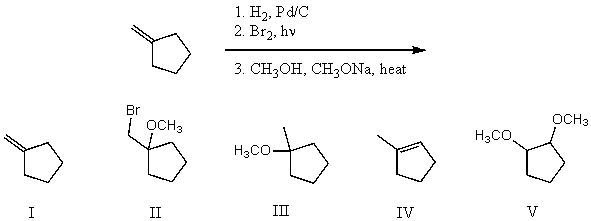
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which hydrogen would be abstracted first when mono-brominating with Br2 and light? 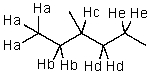
A) Ha
B) Hb
C) Hc
D) Hd
E) He

A) Ha
B) Hb
C) Hc
D) Hd
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
44
Free radical mono-halogenation of an alkane is typically conducted using bromine versus chlorine because
A) the bromine radical is more reactive and therefore more selective.
B) the chlorine radical is more reactive and therefore more selective.
C) the chlorine radical is less reactive and therefore more selective.
D) the bromine radical is less reactive and therefore more selective.
E) None of the above
A) the bromine radical is more reactive and therefore more selective.
B) the chlorine radical is more reactive and therefore more selective.
C) the chlorine radical is less reactive and therefore more selective.
D) the bromine radical is less reactive and therefore more selective.
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
45
Mono-bromination of the following alkane,  , (using Br2 with light)would be?
, (using Br2 with light)would be? 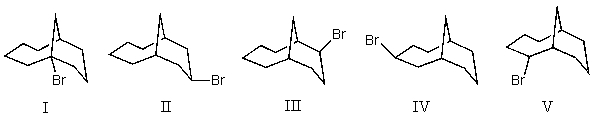
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
46
Mono-bromination of the following alkane, 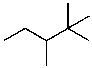 , (using Br2 with light)would be?
, (using Br2 with light)would be? 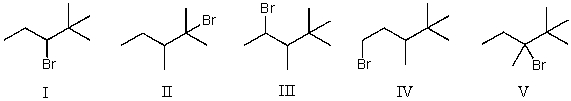
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the product for the following three-step reaction sequence? 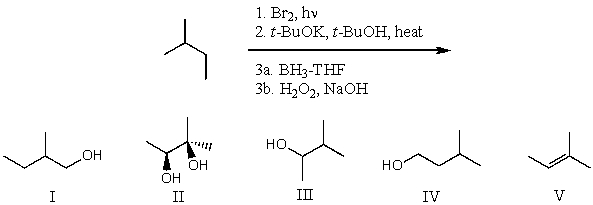
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which hydrogen would be abstracted first when mono-brominating with Br2 and light? 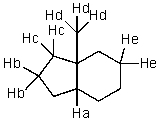
A) Ha
B) Hb
C) Hc
D) Hd
E) He

A) Ha
B) Hb
C) Hc
D) Hd
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the final product,C,obtained via the following reaction sequence? 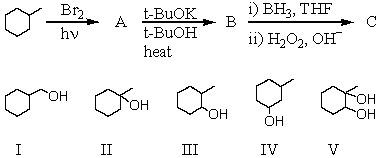
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which hydrogen would be abstracted first when mono-brominating with Br2 and light? 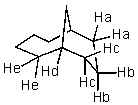
A) Ha
B) Hb
C) Hc
D) Hd
E) He

A) Ha
B) Hb
C) Hc
D) Hd
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
51
Mono-bromination of the following alkane, 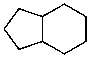 , (using Br2 with light)would be?
, (using Br2 with light)would be? 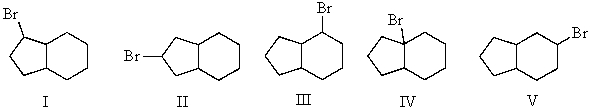
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the product for the following three-step reaction sequence? 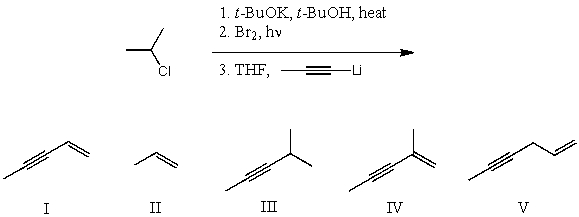
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the product for the following three-step reaction sequence? 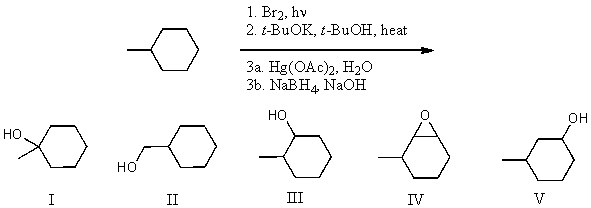
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
54
What is the product for the following three-step reaction sequence? 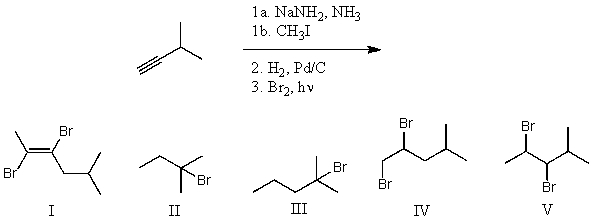
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the product for the following reaction sequence? 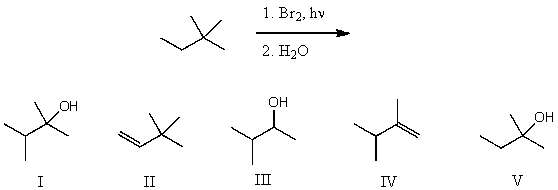
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which hydrogen would be abstracted first when mono-brominating with Br2 and light? 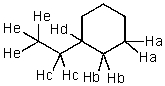
A) Ha
B) Hb
C) Hc
D) Hd
E) He

A) Ha
B) Hb
C) Hc
D) Hd
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
57
Mono-bromination of the following alkane, 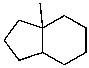 , (using Br2 with light)would be?
, (using Br2 with light)would be? 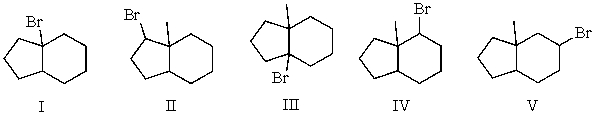
A) I
B) II
C) III
D) IV
E) V
 , (using Br2 with light)would be?
, (using Br2 with light)would be? 
A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the product for the following three-step reaction sequence? 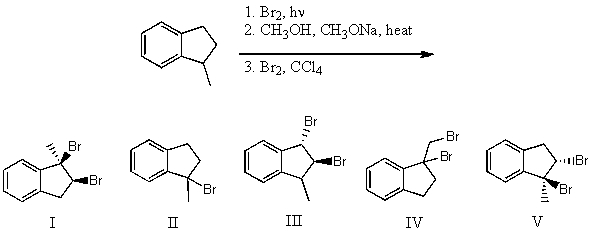
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which hydrogen would be abstracted first when mono-brominating with Br2 and light? 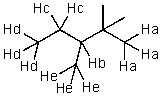
A) Ha
B) Hb
C) Hc
D) Hd
E) He

A) Ha
B) Hb
C) Hc
D) Hd
E) He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the product for the following three-step reaction sequence? 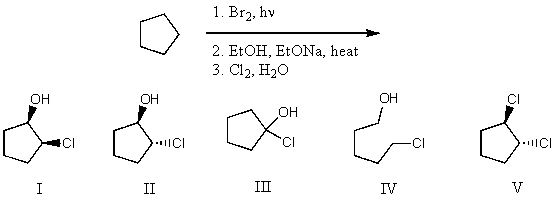
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
61
The free radical chlorination of pentane produces this number of monochloro compounds,including stereoisomers.
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
62
The free radical chlorination of 3-chloropentane forms a mixture of dichloropentanes which,on precise fractional distillation,affords these fractions:
A) 4 fractions,none optically active
B) 4 fractions,2 optically active
C) 7 fractions,4 optically active
D) 7 fractions,6 optically active
E) 7 fractions,all optically active
A) 4 fractions,none optically active
B) 4 fractions,2 optically active
C) 7 fractions,4 optically active
D) 7 fractions,6 optically active
E) 7 fractions,all optically active

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
63
More than one monochloro compound can be obtained from the free radical chlorination of:
A) Cyclopentane
B) Neopentane
C) Isobutane
D) Ethane
E) Methane
A) Cyclopentane
B) Neopentane
C) Isobutane
D) Ethane
E) Methane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
64
The p-orbital of a methyl radical carbon,CH3·,contains how many electrons?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
65
What product would result from the following reaction? 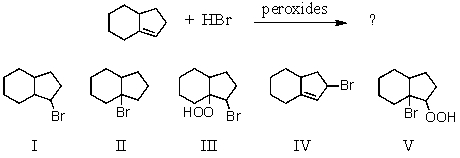
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the total number of trichloropropanes which can be produced by free radical chlorination of propane? Include all stereoisomers.
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the product for the following three-step reaction sequence? 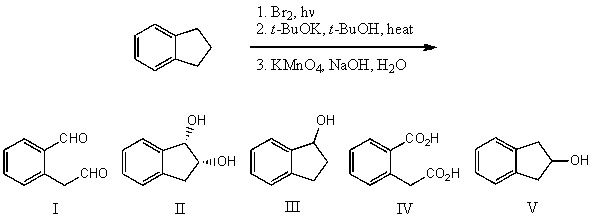
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
68
Free radical chlorination of hexane produces this number of monochloro derivatives (including stereoisomers):
A) 3
B) 4
C) 5
D) 7
E) 8
A) 3
B) 4
C) 5
D) 7
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the product for the following three-step reaction sequence? 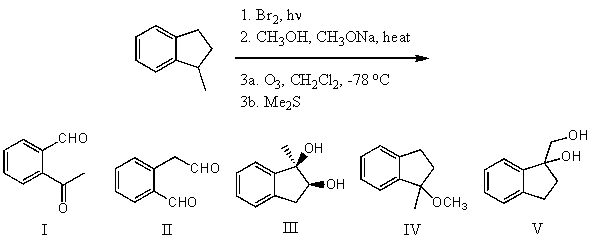
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the product for the following three-step reaction sequence? 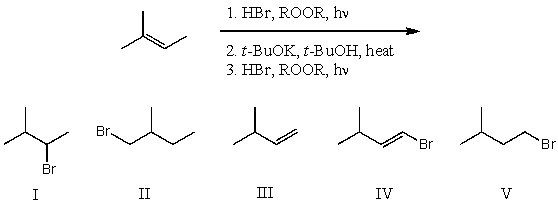
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
71
How many monochloro derivatives,including stereoisomers,can be formed in the chlorination of 1-bromobutane?
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the product for the following three-step reaction sequence? 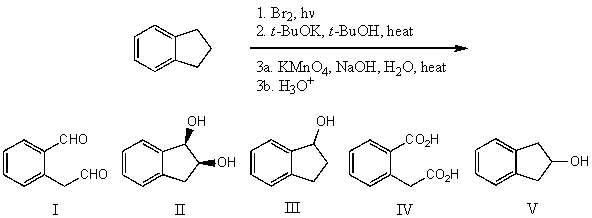
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
73
The reaction of 1-butene with HBr in the presence of peroxides yields 1-bromobutane.The mechanism for the reaction involves:
A) attack on the alkene by a Br+ ion.
B) attack on the alkene by a H+ ion.
C) attack on the alkene by a bromine atom,Br·.
D) attack on the alkene by a hydrogen atom,H·.
E) isomerization of the 2-bromobutane produced initially.
A) attack on the alkene by a Br+ ion.
B) attack on the alkene by a H+ ion.
C) attack on the alkene by a bromine atom,Br·.
D) attack on the alkene by a hydrogen atom,H·.
E) isomerization of the 2-bromobutane produced initially.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the product for the following three-step reaction sequence? 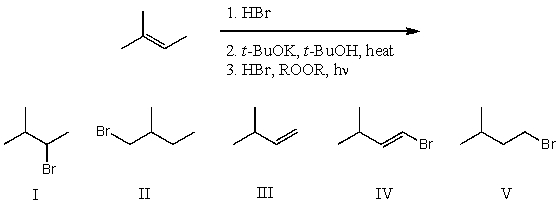
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the product for the following three-step reaction sequence? 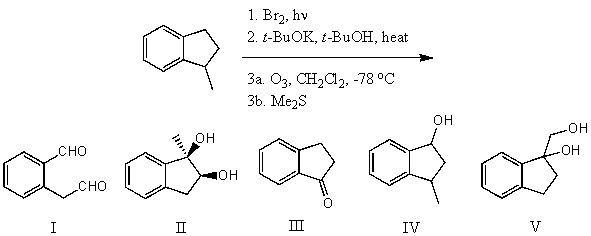
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following would serve as the best synthesis of 2-bromohexane?
A)
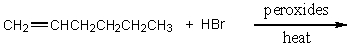
B)
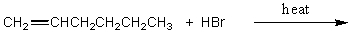
C)
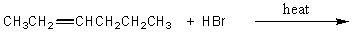
D)
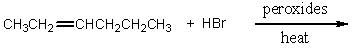
E) All of the above would be equally suitable.
A)
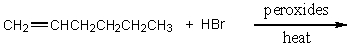
B)
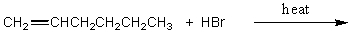
C)
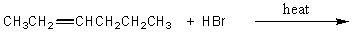
D)
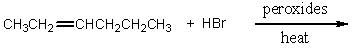
E) All of the above would be equally suitable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
77
The free radical chlorination of (R)-2-chloropentane forms a mixture of dichloropentanes which includes:
A) three optically active compounds.
B) two achiral compounds.
C) two meso compounds.
D) one pair of diastereomers.
E) one racemic mixture.
A) three optically active compounds.
B) two achiral compounds.
C) two meso compounds.
D) one pair of diastereomers.
E) one racemic mixture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
78
Consider the light-initiated chlorination of (S)-2-chlorobutane followed by careful fractional distillation (or separation by GLC)of all of the products with the formula C4H8Cl2.How many fractions (in total)would be obtained and how many of these fractions would be optically active?
A) Three fractions total;all optically active
B) Four fractions total;three fractions optically active
C) Five fractions total;all optically active
D) Five fractions total;four fractions optically active
E) Five fractions total;three fractions optically active
A) Three fractions total;all optically active
B) Four fractions total;three fractions optically active
C) Five fractions total;all optically active
D) Five fractions total;four fractions optically active
E) Five fractions total;three fractions optically active

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
79
Free radical chlorination will produce but one monochloro derivative in the case of:
A) Propane
B) Butane
C) Isobutane
D) Isopentane
E) Neopentane
A) Propane
B) Butane
C) Isobutane
D) Isopentane
E) Neopentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which of the following combinations of reactants can provide a demonstrable example of anti-Markovnikov addition?
A) CH2=CHCH3 + HCl + ROOR
B) CH3CH=CH2 + H2O + Cl2
C) CH3CH=CHCH3 + HBr + ROOR
D) CH3CH2CH=CH2 + HBr + ROOR
E) CH3CH2CH=CH2 + Br2 + ROOR
A) CH2=CHCH3 + HCl + ROOR
B) CH3CH=CH2 + H2O + Cl2
C) CH3CH=CHCH3 + HBr + ROOR
D) CH3CH2CH=CH2 + HBr + ROOR
E) CH3CH2CH=CH2 + Br2 + ROOR

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck








