Deck 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/94
العب
ملء الشاشة (f)
Deck 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination
1
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 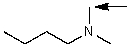
A) 3.00 ppm,doublet
B) 3.00 ppm,triplet
C) 5.00 ppm,triplet
D) 1.00 ppm,doublet
E) 3.00 ppm,singlet
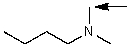
A) 3.00 ppm,doublet
B) 3.00 ppm,triplet
C) 5.00 ppm,triplet
D) 1.00 ppm,doublet
E) 3.00 ppm,singlet
5.00 ppm,triplet
2
How many 1H NMR signals would trans-1,2-dichlorocyclopropane give?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
2
3
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 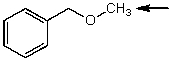
A) 1.10 ppm,singlet
B) 2.10 ppm,doublet
C) 3.40 ppm,singlet
D) 4.5 ppm,singlet
E) 3.5 ppm,quartet

A) 1.10 ppm,singlet
B) 2.10 ppm,doublet
C) 3.40 ppm,singlet
D) 4.5 ppm,singlet
E) 3.5 ppm,quartet
3.40 ppm,singlet
4
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 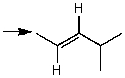
A) 1.00 ppm,doublet
B) 2.00 ppm,singlet
C) 2.00 ppm,triplet
D) 2.00 ppm,doublet
E) 1.00 ppm,triplet

A) 1.00 ppm,doublet
B) 2.00 ppm,singlet
C) 2.00 ppm,triplet
D) 2.00 ppm,doublet
E) 1.00 ppm,triplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
5
For the C2 methylene group in 1-bromopropane,the theoretical multiplicity in the 1H NMR spectrum,presuming that Jab is sufficiently different from Jbc and that the instrument has sufficient resolving power,is which of these? 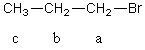
A) 2
B) 5
C) 6
D) 8
E) 12
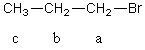
A) 2
B) 5
C) 6
D) 8
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
6
A downfield ( 9-10)singlet is observed in the 1H NMR spectrum of:
A)
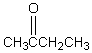
B)
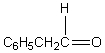
C)
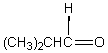
D)
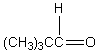
E)
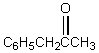
A)
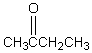
B)
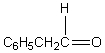
C)
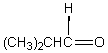
D)
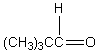
E)
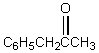

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 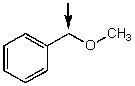
A) 1.10 ppm,singlet
B) 2.10 ppm,triplet
C) 3.40 ppm,triplet
D) 4.5 ppm,singlet
E) 5.3 ppm,doublet

A) 1.10 ppm,singlet
B) 2.10 ppm,triplet
C) 3.40 ppm,triplet
D) 4.5 ppm,singlet
E) 5.3 ppm,doublet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 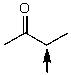
A) 1.00 ppm,quartet
B) 2.40 ppm,singlet
C) 2.40 ppm,quartet
D) 3.00 ppm,quartet
E) 2.40 ppm,triplet

A) 1.00 ppm,quartet
B) 2.40 ppm,singlet
C) 2.40 ppm,quartet
D) 3.00 ppm,quartet
E) 2.40 ppm,triplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 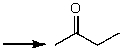
A) 1.00 ppm,singlet
B) 2.10 ppm,singlet
C) 2.10 ppm,quartet
D) 3.00 ppm,singlet
E) 2.10 ppm,triplet

A) 1.00 ppm,singlet
B) 2.10 ppm,singlet
C) 2.10 ppm,quartet
D) 3.00 ppm,singlet
E) 2.10 ppm,triplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 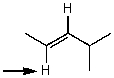
A) 5.40 ppm,multiplet
B) 2.00 ppm,multiplet
C) 2.00 ppm,doublet
D) 2.00 ppm,quartet
E) 5.40 ppm,doublet

A) 5.40 ppm,multiplet
B) 2.00 ppm,multiplet
C) 2.00 ppm,doublet
D) 2.00 ppm,quartet
E) 5.40 ppm,doublet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many chemically distinct 1H NMR signals are there in the following compound? 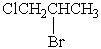
A) 1
B) 2
C) 3
D) 4
E) 5
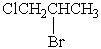
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many 1H NMR signals would cis-1,2-dichlorocyclopropane give?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
13
An organic compound absorbs strongly in the IR at 1687 cm-1.Its 1H NMR spectrum consists of two signals,a singlet at 2.1 ppm and a multiplet centered at 7.1 ppm.Its mass spectrum shows significant peaks at m/z 120,m/z 105 and m/z 77.This information is consistent with which of the following structures? 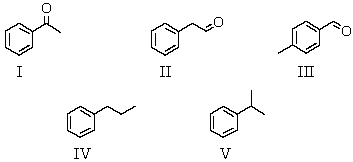
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which proton(s)of the compound below would appear as a septet in the 1H NMR spectrum? 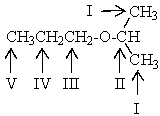
A) The protons on carbon I
B) The protons on carbon II
C) The protons on carbon III
D) The protons on carbon IV
E) The protons on carbon V

A) The protons on carbon I
B) The protons on carbon II
C) The protons on carbon III
D) The protons on carbon IV
E) The protons on carbon V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
15
The 1H NMR signal for which of the indicated protons occurs farthest downfield? 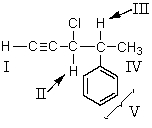
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 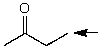
A) 1.00 ppm,quartet
B) 2.40 ppm,singlet
C) 2.40 ppm,quartet
D) 3.00 ppm,quartet
E) 1.00 ppm,triplet
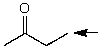
A) 1.00 ppm,quartet
B) 2.40 ppm,singlet
C) 2.40 ppm,quartet
D) 3.00 ppm,quartet
E) 1.00 ppm,triplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 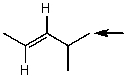
A) 5.40 ppm,doublet
B) 1.00 ppm,multiplet
C) 2.00 ppm,doublet
D) 1.00 ppm,doublet
E) 5.40 ppm,multiplet

A) 5.40 ppm,doublet
B) 1.00 ppm,multiplet
C) 2.00 ppm,doublet
D) 1.00 ppm,doublet
E) 5.40 ppm,multiplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
18
If all the protons of 1-fluoropentane could be discerned,which would you expect to be at the lowest field in the 1H NMR spectrum of this compound? 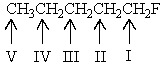
A) Protons on carbon I
B) Protons on carbon II
C) Protons on carbon III
D) Protons on carbon IV
E) Protons on carbon V
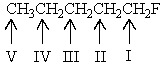
A) Protons on carbon I
B) Protons on carbon II
C) Protons on carbon III
D) Protons on carbon IV
E) Protons on carbon V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s)indicated with the arrow? 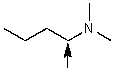
A) 3.00 ppm,doublet
B) 3.00 ppm,triplet
C) 5.00 ppm,triplet
D) 1.00 ppm,doublet
E) 5.40 ppm,multiplet

A) 3.00 ppm,doublet
B) 3.00 ppm,triplet
C) 5.00 ppm,triplet
D) 1.00 ppm,doublet
E) 5.40 ppm,multiplet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
20
The 1H NMR spectrum of which of these compounds would consist of a triplet,singlet and quartet only?
A) 2-chloro-4-methylpentane
B) 3-chloro-2-methylpentane
C) 3-chloropentane
D) 1-chloro-2,2-dimethylbutane
E) 3-chloro-3-methylpentane
A) 2-chloro-4-methylpentane
B) 3-chloro-2-methylpentane
C) 3-chloropentane
D) 1-chloro-2,2-dimethylbutane
E) 3-chloro-3-methylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
21
A compound with the molecular formula C6H15N gave the following 1H NMR spectrum: triplet, 0.90
Quartet, 2.4
There were no other signals.The most likely structure for the compound is:
A)
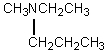
B)
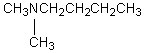
C) CH3CH2CH2CH2CH2CH2NH2
D)
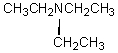
E)
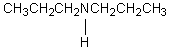
Quartet, 2.4
There were no other signals.The most likely structure for the compound is:
A)
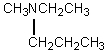
B)
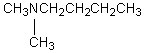
C) CH3CH2CH2CH2CH2CH2NH2
D)
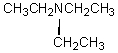
E)
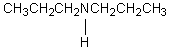

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
22
The 1H NMR spectrum of which of the compounds below,all of formula C7H12O2,would consist of two singlets only? 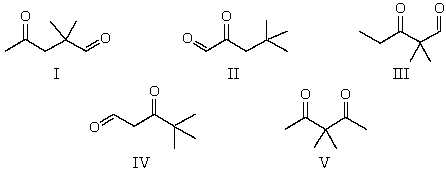
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which proton(s)of the compound below would appear as a doublet in the 1H NMR spectrum? 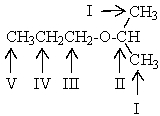
A) The protons on carbon I
B) The protons on carbon II
C) The protons on carbon III
D) The protons on carbon IV
E) The protons on carbon V

A) The protons on carbon I
B) The protons on carbon II
C) The protons on carbon III
D) The protons on carbon IV
E) The protons on carbon V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
24
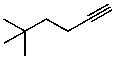
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C8H14? Relative integration is shown. 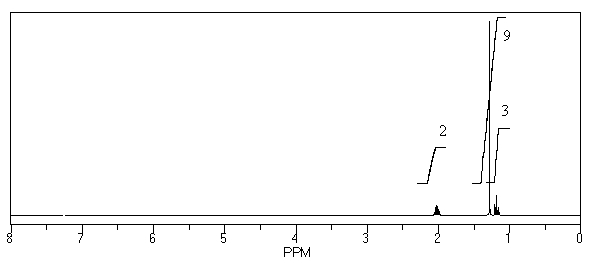
A)
B)

C)
D)

E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
25
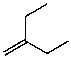
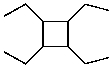
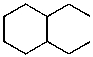
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C10H20? Relative integration is shown. 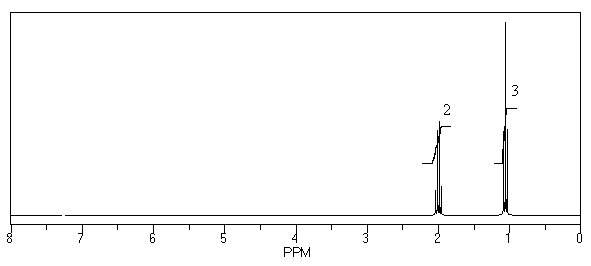
A)
B)
C)
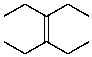
D)
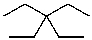
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
26
The 1H NMR spectrum of which of the compounds below,all of formula C7H12O2,would consist of three singlets only? 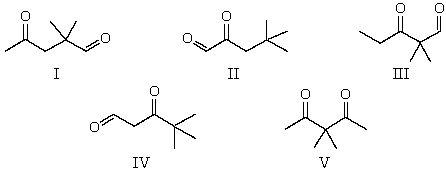
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
27
A compound with the molecular formula C10H13Cl gave the following 1H NMR spectrum: singlet, 1.6
Singlet, 3.1
Multiplet, 7.2 (5H)
The most likely structure for the compound is: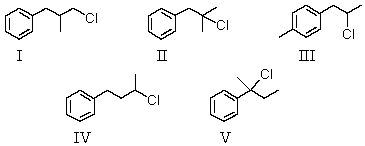
A) I
B) II
C) III
D) IV
E) V
Singlet, 3.1
Multiplet, 7.2 (5H)
The most likely structure for the compound is:

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H14O? IR data shows no characteristic peak around 1700 cm-1.The 13C-NMR chemical shifts (ppm): 108.4,50.9,31.6,23.5,2.0.Relative integration is known. 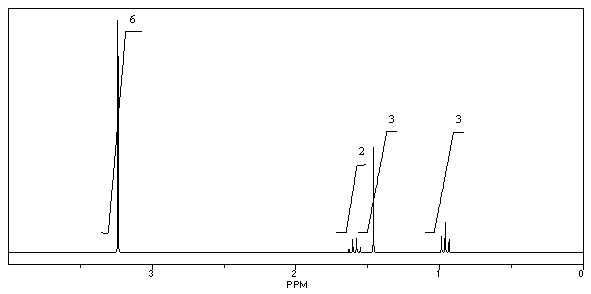
A)
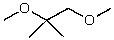
B)
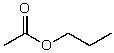
C)
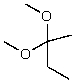
D)
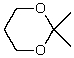
E)
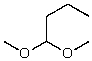

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
29
Predict the splitting pattern you would observe for the proton at C3 of 2,3-dimethyl-2-phenylbutane. 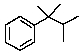
A) Doublet
B) Singlet
C) Quartet
D) Septet
E) Octet

A) Doublet
B) Singlet
C) Quartet
D) Septet
E) Octet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of these compounds will not be represented by a singlet only in the 1H NMR spectrum?
A) Neopentane
B) Hexamethylbenzene
C) Isobutane
D) (Z)-1,2-Dichloroethene
E) (E)-1,2-Dichloroethene
A) Neopentane
B) Hexamethylbenzene
C) Isobutane
D) (Z)-1,2-Dichloroethene
E) (E)-1,2-Dichloroethene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
31
A compound with the molecular formula C8H9ClO gave the following 1H NMR spectrum: triplet, 3.7
Triplet, 4.2
Multiplet 7.1
There was no evidence of an -OH band in the IR spectrum.The most likely structure for the compound is: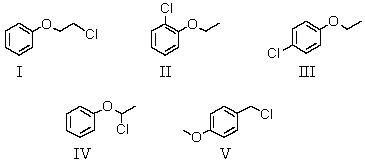
A) I
B) II
C) III
D) IV
E) V
Triplet, 4.2
Multiplet 7.1
There was no evidence of an -OH band in the IR spectrum.The most likely structure for the compound is:

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
32
A compound with the molecular formula C4H10O gives a 1H NMR spectrum consisting only of a quartet centered at 3.5 and a triplet at 1.1.The most likely structure for the compound is:
A)
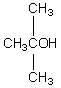
B)
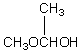
C) CH3CH2CH2CH2OH
D) CH3CH2OCH2CH3
E)
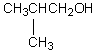
A)

B)

C) CH3CH2CH2CH2OH
D) CH3CH2OCH2CH3
E)
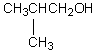

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
33
A compound with the molecular formula C8H9BrO gave the following 1H NMR spectrum: triplet, 1.4
Quartet, 3.9
Multiplet, 7.0 (4H)
There was no evidence of an -OH band in the IR spectrum.A possible structure for the compound is: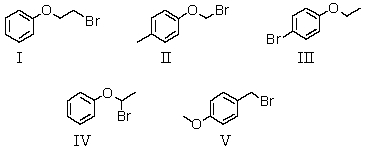
A) I
B) II
C) III
D) IV
E) V
Quartet, 3.9
Multiplet, 7.0 (4H)
There was no evidence of an -OH band in the IR spectrum.A possible structure for the compound is:

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
34
Consider the expected splitting of signal "b" in the 1H NMR spectrum of 1-methoxy-2-methylpropane,shown below.Presuming that Jab is sufficiently different from Jbc and that the instrument has sufficient resolving power,what is the theoretical multiplicity of signal "b"? 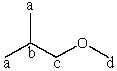
A) 8
B) 9
C) 12
D) 21
E) 24

A) 8
B) 9
C) 12
D) 21
E) 24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
35
A compound with the molecular formula C3H6Cl2 gave a 1H NMR spectrum consisting only of a triplet centered at 3.7 and a quintet centered at 2.2.The most likely structure for the compound is:
A) CH3CH2CHCl2
B) CH3CHClCH2Cl
C) ClCH2CHClCH3
D) ClCH2CH2CH2Cl
E) CH3CCl2CH3
A) CH3CH2CHCl2
B) CH3CHClCH2Cl
C) ClCH2CHClCH3
D) ClCH2CH2CH2Cl
E) CH3CCl2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
36
Determine the most likely structure of a compound,with the molecular formula C9H12,which gave a 1H NMR spectrum consisting of: a doublet at 1.25
A septet at 2.90 and
A multiplet at 7.25![<strong>Determine the most likely structure of a compound,with the molecular formula C<sub>9</sub>H<sub>12</sub>,which gave a <sup>1</sup>H NMR spectrum consisting of: a doublet at \delta 1.25 A septet at \delta 2.90 and A multiplet at \delta 7.25 ]</strong> A) I B) II C) III D) IV E) V](https://d2lvgg3v3hfg70.cloudfront.net/TB5901/11ea9a02_1b24_5831_8bb6_315348c46757_TB5901_00.jpg) ]
]
A) I
B) II
C) III
D) IV
E) V
A septet at 2.90 and
A multiplet at 7.25
![<strong>Determine the most likely structure of a compound,with the molecular formula C<sub>9</sub>H<sub>12</sub>,which gave a <sup>1</sup>H NMR spectrum consisting of: a doublet at \delta 1.25 A septet at \delta 2.90 and A multiplet at \delta 7.25 ]</strong> A) I B) II C) III D) IV E) V](https://d2lvgg3v3hfg70.cloudfront.net/TB5901/11ea9a02_1b24_5831_8bb6_315348c46757_TB5901_00.jpg) ]
]A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H11N? In the IR spectrum you notice a stretch at about 2250 cm-1.Relative integration is shown. 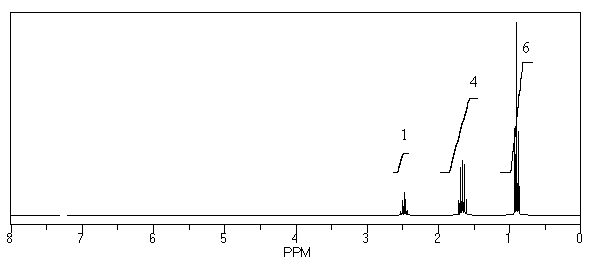
A)

B)
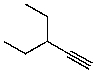
C)
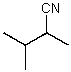
D)
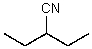
E)
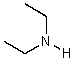

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H10O2? Relative integration is shown. 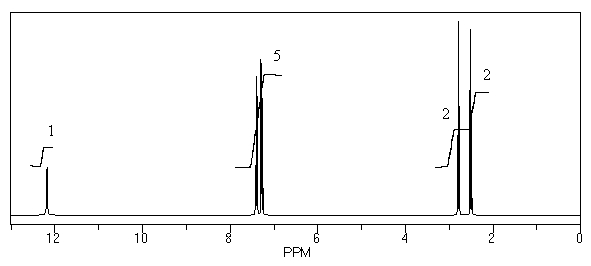
A)
B)
C)
D)
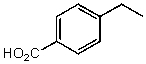
E)
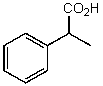

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which proton(s)of the compound below would appear as a triplet in the 1H NMR spectrum? 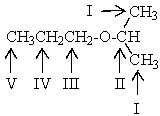
A) The protons on carbon II
B) The protons on carbon I and V
C) The protons on carbon III and V
D) The protons on carbon III and IV
E) The protons on carbon V

A) The protons on carbon II
B) The protons on carbon I and V
C) The protons on carbon III and V
D) The protons on carbon III and IV
E) The protons on carbon V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
40
The 1H NMR spectrum of which of the compounds below,all of formula C7H12O2,would consist of a singlet,a doublet and a triplet only? 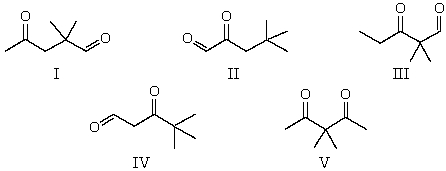
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H10O2? Relative integration is shown. 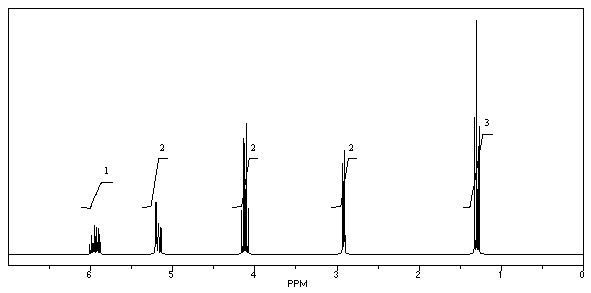
A)
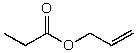
B)
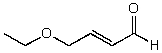
C)
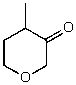
D)
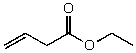
E)
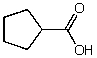

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H10O2? The 13C-NMR shows characteristic chemical shifts at 22.3,31.1,117.3,157.7,and 171.6 ppm.Relative integration is shown. 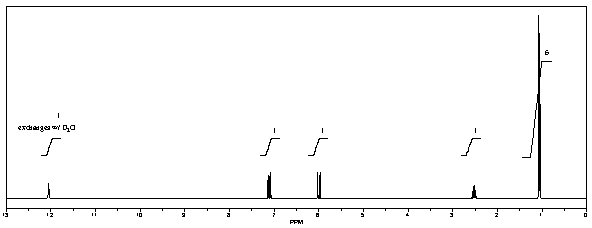
A)
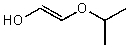
B)
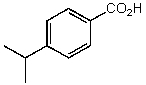
C)
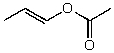
D)
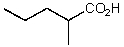
E)
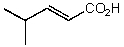

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
43
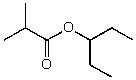
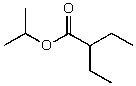
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H18O2 and characteristic 13C-NMR peaks at 11.3,21.6,25.3,49.4,67.1,and 175.5 ppm? Relative integration is shown. 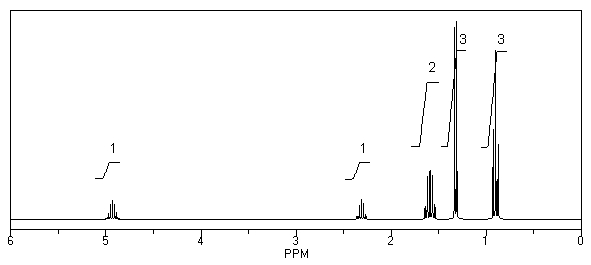
A)
B)
C)
D)
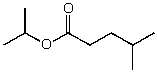
E)
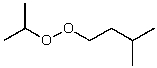

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12? Relative integration is shown. 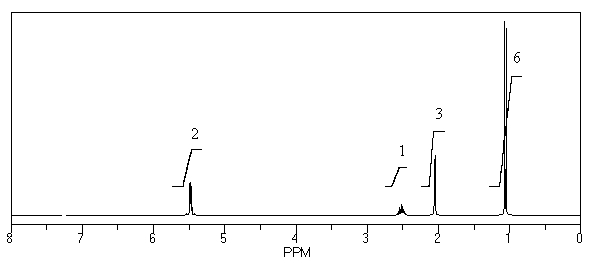
A)
B)
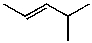
C)
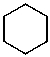
D)
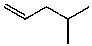
E)


A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12O2? Relative integration is shown. 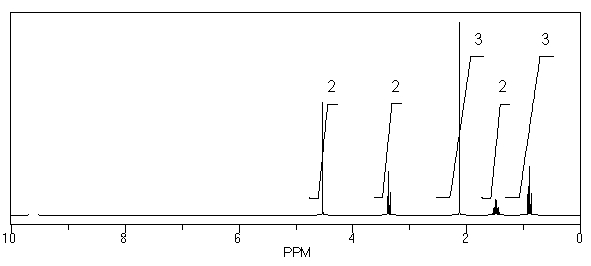
A)
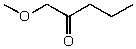
B)
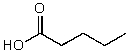
C)
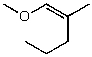
D)
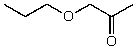
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
46
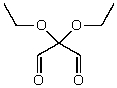
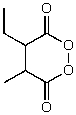
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C7H12O4? The 13C-NMR spectrum shows peaks at 14.1,40.8,61.0 and 166.8 ppm.Relative integration is shown. 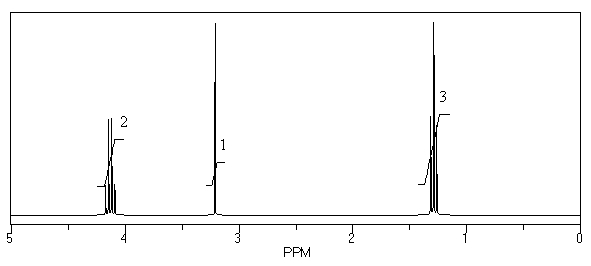
A)
B)
C)
D)
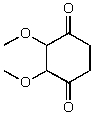
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
47
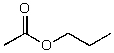
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C6H12O which shows no characteristic stretches in the IR between 3600-3300 cm-1,but does around 1600 cm-1? Relative integration is shown. 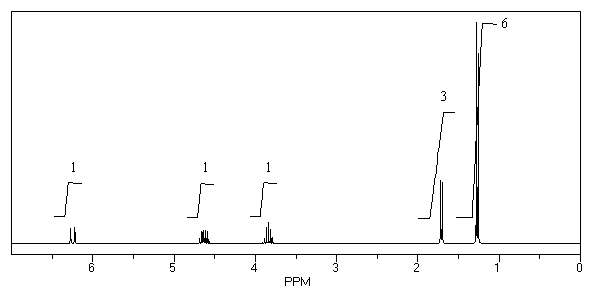
A)
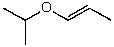
B)
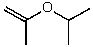
C)
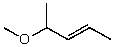
D)
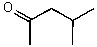
E)
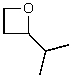

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C8H15ClO3,which shows a characteristic stretch in the IR around 1750 cm-1 but not around 3500 cm-1,and a characteristic peak at 173 ppm in the 13C-NMR? Relative integration is shown. 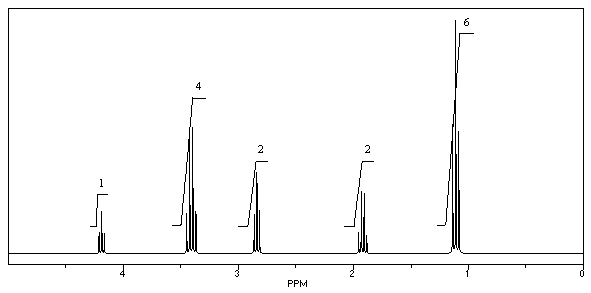
A)
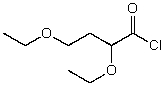
B)
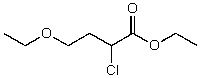
C)
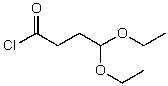
D)
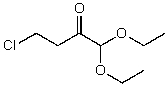
E)
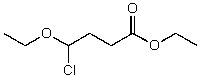

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H12O? Relative integration is shown. 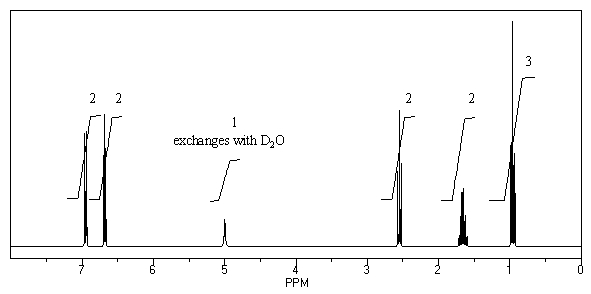
A)
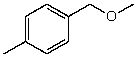
B)
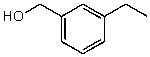
C)
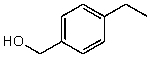
D)
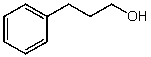
E)
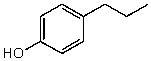

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
50
A compound C4H9Br gave the following 1H NMR spectrum: triplet, 1.0 (3H);doublet, 1.7;multiplet, 1.8;multiplet, 4.1 (1H)
Which is a reasonable structure for the compound?
A) CH3CH2CHBrCH3
B) CH3CH2CH2CH2Br
C) (CH3)2CHCH2Br
D) (CH3)3CBr
Which is a reasonable structure for the compound?
A) CH3CH2CHBrCH3
B) CH3CH2CH2CH2Br
C) (CH3)2CHCH2Br
D) (CH3)3CBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C8H16O? Relative integration is shown. 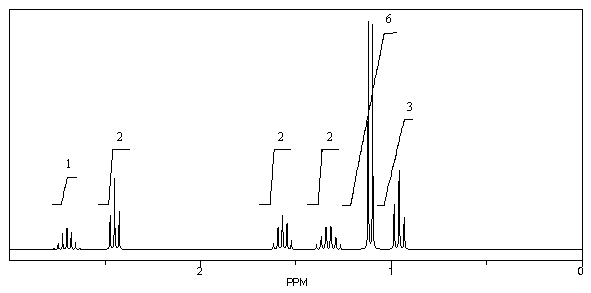
A)
B)
C)
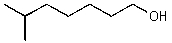
D)
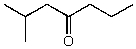
E)
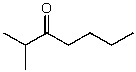

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C10H12O2? Relative integration is shown. 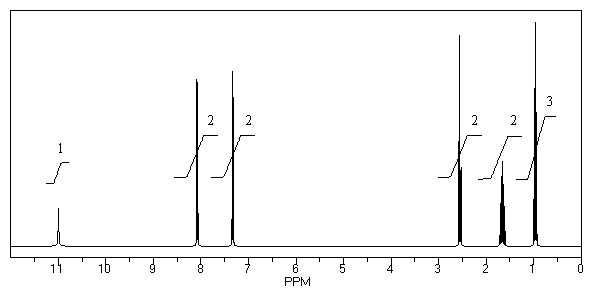
A)
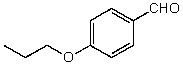
B)
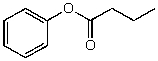
C)
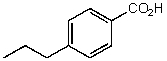
D)
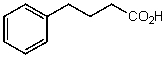
E)
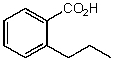

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H8O? The IR spectrum does show a characteristic stretch around 1700 cm-1.Relative integration is shown. 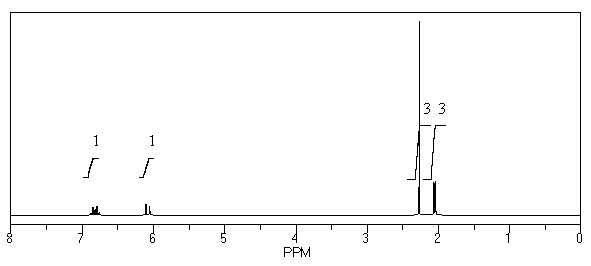
A)
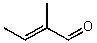
B)
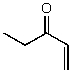
C)
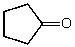
D)
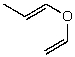
E)
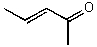

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
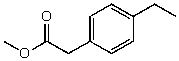
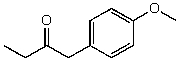
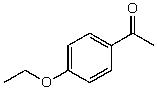
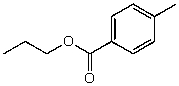
54
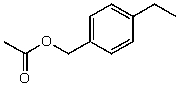
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C11H14O2? Looking at the 13C-NMR you notice a peak at 174 ppm.Relative integration is shown. 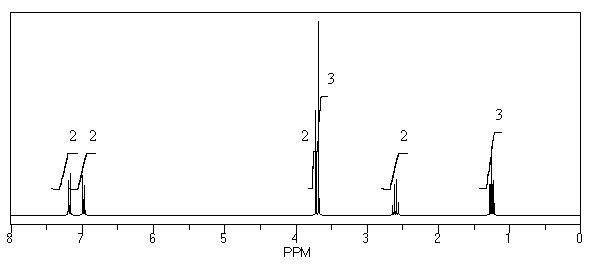
A)
B)
C)
D)
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
55
A compound C5H10O gave the following spectral data: 1H NMR spectrum IR spectrum
Doublet, 1.10 strong peak
Singlet, 2.10 near 1720 cm-1
Septet, 2.50
Which is a reasonable structure for the compound?
A) I
B) II
C) III
D) IV
E) V
Doublet, 1.10 strong peak
Singlet, 2.10 near 1720 cm-1
Septet, 2.50
Which is a reasonable structure for the compound?

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H10O? The IR spectrum does not show any characteristic stretches around 1700 cm-1.Relative integration is shown. 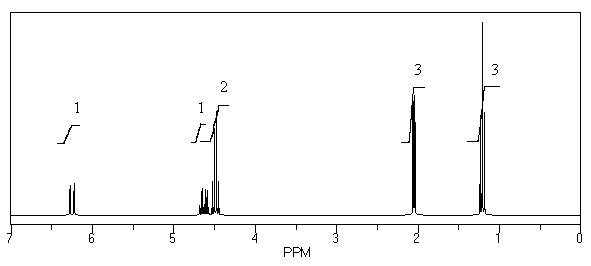
A)
B)
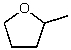
C)
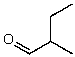
D)
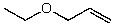
E)


A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
57
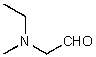
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H11NO,which shows a characteristic stretch in the IR around 1700 cm-1,and a characteristic peak at 202 ppm in the 13C-NMR? Relative integration is shown. 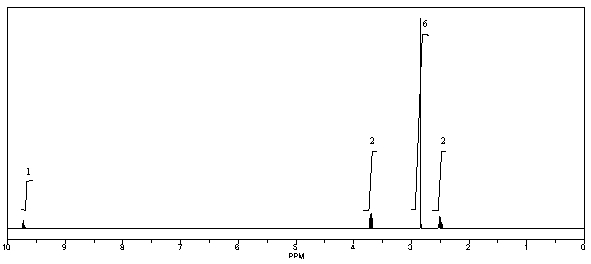
A)
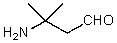
B)
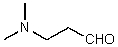
C)
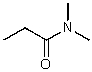
D)
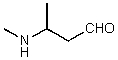
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
58
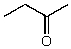
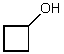
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C4H8O? Relative integration is shown. 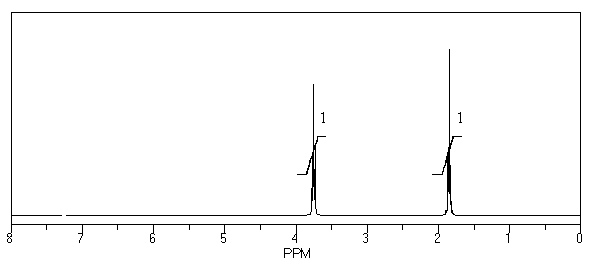
A)
B)

C)
D)
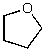
E)
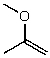

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
59
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C4H7Br? Relative integration is shown. 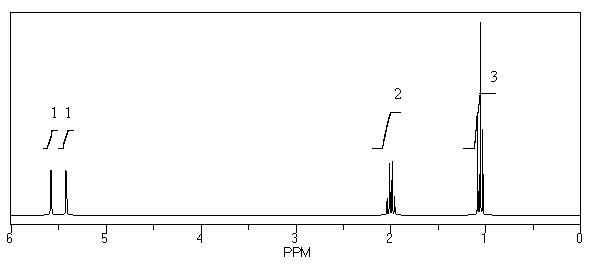
A)

B)

C)
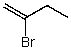
D)

E)
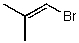

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C10H11N? Looking at the 13C-NMR you notice 10 distinct peaks,and the IR has a characteristic peak around 2250 cm-1.Relative integration is shown. 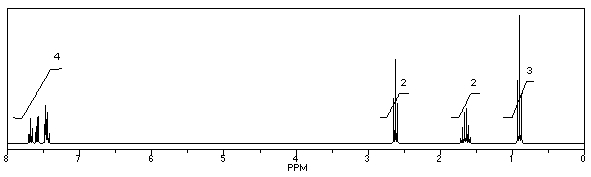
A)
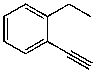
B)
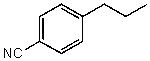
C)
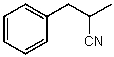
D)
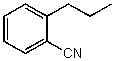
E)
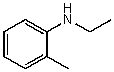

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
61
In NMR terminology,protons Ha and Hb are said to be: 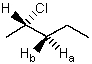
A) Identical
B) Enantiotopic
C) Diastereotopic
D) Homotopic
E) Mesotopic

A) Identical
B) Enantiotopic
C) Diastereotopic
D) Homotopic
E) Mesotopic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
62
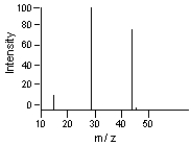
-Which is the likely molecular ion (M - )?
A) 15
B) 29
C) 44
D) 45
E) 100

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
63
How many 13C signals would you expect from anisole? 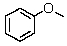
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
64
How will the methyl carbon appear in the proton off-resonance decoupled 13C spectrum of toluene? 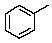
A) Singlet
B) Doublet
C) Triplet
D) Quartet
E) Quintet

A) Singlet
B) Doublet
C) Triplet
D) Quartet
E) Quintet

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
65
Determine the likely structure for a compound A (C6H10O),which is found to decolorize bromine in carbon tetrachloride.Its spectral data is as follows: 1H NMR IR
Triplet, 1.0 singlet, 2.4 2200 cm-1 (sharp)
Singlet, 1.4 singlet, 3.4 3300 cm-1 (sharp)
Quartet, 1.6 3500 cm-1 (broad)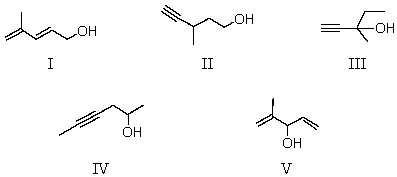
A) I
B) II
C) III
D) IV
E) V
Triplet, 1.0 singlet, 2.4 2200 cm-1 (sharp)
Singlet, 1.4 singlet, 3.4 3300 cm-1 (sharp)
Quartet, 1.6 3500 cm-1 (broad)

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
66
How many 13C signals would 1,4-dimethylbenzene give? 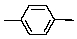
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
67
A prominent (M 1+ -18)peak suggests that the compound might be a(n):
A) Alkane
B) Alcohol
C) Ether
D) Ketone
E) Primary amine
A) Alkane
B) Alcohol
C) Ether
D) Ketone
E) Primary amine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
68
How many 13C signals would 1,3-dichlorobenzene give? 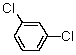
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
69
The broadband proton-decoupled 13C NMR spectrum of a hexyl chloride exhibits five signals.Which of these structures could be the correct one for the compound?
A)
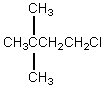
B)
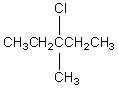
C)
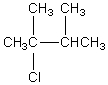
D)
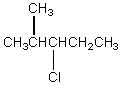
E)
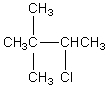
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
70
How many signals will be recorded in the broadband proton-decoupled 13C spectrum of 4-chloro-1-ethylbenzene? 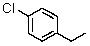
A) 2
B) 3
C) 4
D) 6
E) 7

A) 2
B) 3
C) 4
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
71
Select the structure of a compound C6H14 with a base peak at m/z 43.
A) CH3CH2CH2CH2CH2CH3
B) (CH3CH2)2CHCH3
C) (CH3)3CCH2CH3
D) (CH3)2CHCH(CH3)2
E) None of these
A) CH3CH2CH2CH2CH2CH3
B) (CH3CH2)2CHCH3
C) (CH3)3CCH2CH3
D) (CH3)2CHCH(CH3)2
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
72
A compound with the molecular formula C10H14 gave the following 1H NMR spectrum: doublet, 1.2
Singlet, 2.3
Septet, 2.8
Multiplet, 7.1
A possible structure for the compound is: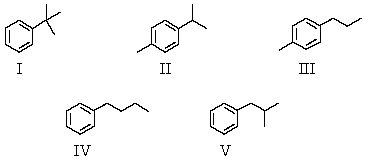
A) I
B) II
C) III
D) IV
E) V
Singlet, 2.3
Septet, 2.8
Multiplet, 7.1
A possible structure for the compound is:

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
73
The C7 compound which gives 3 signals in the broadband proton-decoupled 13C spectrum could be:
A) Heptane
B) 2-Methylhexane
C) 3,3-Dimethylpentane
D) 2,4-Dimethylpentane
E) 2,2,3-Trimethylbutane
A) Heptane
B) 2-Methylhexane
C) 3,3-Dimethylpentane
D) 2,4-Dimethylpentane
E) 2,2,3-Trimethylbutane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
74
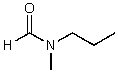
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H11NO? In the 13C-NMR spectrum you notice that the farthest peak downfield has a chemical shift of 173 ppm.Relative integration is shown. 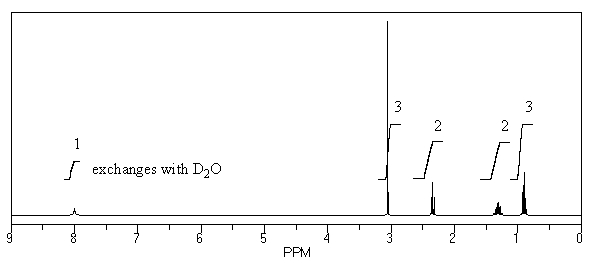
A)
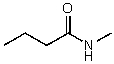
B)
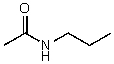
C)
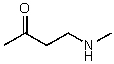
D)
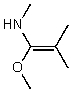
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the molecular formula of this compound? m/z
Intensity
78 M +
10)00
79
1
80
3)3
81
0)3
A) C6H6
B) C3H5Cl
C) C6H8
D) C6H9
E) C3H7Cl
Intensity
78 M +
10)00
79
1
80
3)3
81
0)3
A) C6H6
B) C3H5Cl
C) C6H8
D) C6H9
E) C3H7Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
76
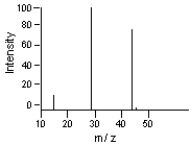
Which is the base peak?
A) 15
B) 29
C) 44
D) 45
E) 100

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
77
In the structure shown,Hb and Hc are classified as: 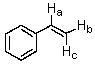
A) homotopic protons.
B) geminal protons.
C) enantiotopic protons.
D) diastereotopic protons.
E) isomeric protons.

A) homotopic protons.
B) geminal protons.
C) enantiotopic protons.
D) diastereotopic protons.
E) isomeric protons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
78
What is the molecular formula of this compound? m/z
Intensity
84 M +
10)00
85
0)56
86
0)04
A) C5H10O
B) C5H8O
C) C5H24
D) C6H12
E) C4H6O2
Intensity
84 M +
10)00
85
0)56
86
0)04
A) C5H10O
B) C5H8O
C) C5H24
D) C6H12
E) C4H6O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
79
How many 13C signals would 1,2-dimethylbenzene give? 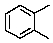
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck
80
A bromodichlorobenzene which gives four signals in the broadband proton-decoupled 13C spectrum could be: 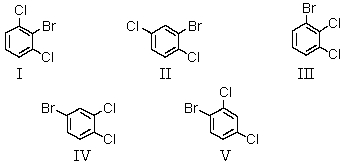
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 94 في هذه المجموعة.
فتح الحزمة
k this deck








