Deck 14: Aromatic Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/151
العب
ملء الشاشة (f)
Deck 14: Aromatic Compounds
1
Which of the following is NOT 2-bromo-5-nitrobenzoic acid? 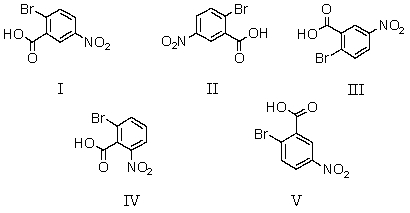
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
IV
2
Which reagent(s)would serve as the basis for a simple chemical test that would distinguish between naphthalene and 2,4,6-decatriene?
A) NaOH in H2O
B) KMnO4,OH - ,H2O
C) NaBH4,H2O
D) H2CrO4
E) None of these
A) NaOH in H2O
B) KMnO4,OH - ,H2O
C) NaBH4,H2O
D) H2CrO4
E) None of these
KMnO4,OH - ,H2O
3
Which reagent(s)would serve as the basis for a simple chemical test that would distinguish between benzene and cyclohexene?
A) NaOH in H2O
B) Br2 in CCl4
C) AgNO3 in C2H5OH
D) NaHSO3 in H2O
E) None of these
A) NaOH in H2O
B) Br2 in CCl4
C) AgNO3 in C2H5OH
D) NaHSO3 in H2O
E) None of these
Br2 in CCl4
4
Phenol is the name commonly assigned to:
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which dibromobenzene can,in theory,yield three mononitro derivatives?
A) o-Dibromobenzene
B) m-Dibromobenzene
C) p-Dibromobenzene
D) All of these
E) None of these
A) o-Dibromobenzene
B) m-Dibromobenzene
C) p-Dibromobenzene
D) All of these
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
6
In theory,a single molecule of this compound will rotate plane-polarized light.
A) Butylbenzene
B) Isobutylbenzene
C) sec-Butylbenzene
D) tert-Butylbenzene
E) None of these
A) Butylbenzene
B) Isobutylbenzene
C) sec-Butylbenzene
D) tert-Butylbenzene
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
7
Xylene is the name commonly assigned to:
A) Hydroxybenzene
B) Aminobenzene
C) Dimethylbenzene
D) Ethylbenzene
E) Methoxybenzene
A) Hydroxybenzene
B) Aminobenzene
C) Dimethylbenzene
D) Ethylbenzene
E) Methoxybenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
8
Toluene is the name commonly assigned to:
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which dibromobenzene can yield only one mononitro derivative?
A) o-Dibromobenzene
B) m-Dibromobenzene
C) p-Dibromobenzene
D) More than one of these
E) None of these
A) o-Dibromobenzene
B) m-Dibromobenzene
C) p-Dibromobenzene
D) More than one of these
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which dihydroxybenzene can yield only one mononitro derivative?
A) o-Dihydroxybenzene
B) m-Dihydroxybenzene
C) p- Dihydroxybenzene
D) More than one of these
E) None of these
A) o-Dihydroxybenzene
B) m-Dihydroxybenzene
C) p- Dihydroxybenzene
D) More than one of these
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which reagent(s)would serve as the basis for a simple chemical test that would distinguish between ethylbenzene and vinylcyclohexane?
A) H2CrO4
B) LAH
C) NaBH4,H2O
D) O3/Zn,H+
E) Two of the above
A) H2CrO4
B) LAH
C) NaBH4,H2O
D) O3/Zn,H+
E) Two of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
12
If thiophene is an aromatic molecule and reacts similarly to benzene,how many (neutral)monobromothiophenes could be obtained in the following reaction? 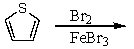
A) 1
B) 2
C) 3
D) 4
E) 5
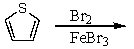
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many different dibromophenols are possible?
A) 8
B) 7
C) 6
D) 5
E) 4
A) 8
B) 7
C) 6
D) 5
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
14
Aniline is the name commonly assigned to:
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which reagent(s)would serve as the basis for a simple chemical test that would distinguish between ethylbenzene and vinylcyclohexane?
A) H2CrO4
B) LAH
C) NaBH4,H2O
D) KMnO4,OH - ,H2O
E) Two of the above
A) H2CrO4
B) LAH
C) NaBH4,H2O
D) KMnO4,OH - ,H2O
E) Two of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which reagent(s)would serve as the basis for a simple chemical test that would distinguish between benzene and 1-hexene?
A) NaOH in H2O
B) Br2 in CCl4
C) AgNO3 in C2H5OH
D) NaHSO3 in H2O
E) None of these
A) NaOH in H2O
B) Br2 in CCl4
C) AgNO3 in C2H5OH
D) NaHSO3 in H2O
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
17
Anisole is the name commonly assigned to:
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene
A) Hydroxybenzene
B) Aminobenzene
C) Methylbenzene
D) Ethylbenzene
E) Methoxybenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
18
The correct name for the compound shown below is: 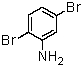
A) 3,4-Dibromoaniline
B) 2,4-Dibromoaniline
C) 2,5-Dibromoaniline
D) 3,6-Dibromoaniline
E) 2,6-Dibromoaniline

A) 3,4-Dibromoaniline
B) 2,4-Dibromoaniline
C) 2,5-Dibromoaniline
D) 3,6-Dibromoaniline
E) 2,6-Dibromoaniline

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which is the only one of these reagents which will react with benzene under the specified conditions?
A) Cl2,FeCl3,heat
B) H2,25°C
C) Br2/CCl4,25°C,dark
D) KMnO4/H2O,25°C
E) H3O+,heat
A) Cl2,FeCl3,heat
B) H2,25°C
C) Br2/CCl4,25°C,dark
D) KMnO4/H2O,25°C
E) H3O+,heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which dichlorobenzene might theoretically yield three mononitro products?
A) o-Dichlorobenzene
B) m-Dichlorobenzene
C) p-Dichlorobenzene
D) None of these
E) All of these
A) o-Dichlorobenzene
B) m-Dichlorobenzene
C) p-Dichlorobenzene
D) None of these
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
21
In which of the following compounds would the longest carbon-carbon bond(s)be found?
A) 2-Bromobenzaldehyde
B) Vinylbenzene
C) 1,3,5-Heptatriene
D) 2,4,6-Octatriene
E) 2-Ethylbenzoic acid
A) 2-Bromobenzaldehyde
B) Vinylbenzene
C) 1,3,5-Heptatriene
D) 2,4,6-Octatriene
E) 2-Ethylbenzoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
22
Of Hückel's requirements for aromatic character,only this one is waived in the case of certain compounds considered to be aromatic.
A) The ring system must be planar.
B) The system must be monocyclic.
C) There must be (4n + 2) electrons.
D) The Hückel number of electrons must be completely delocalized.
E) None.All of these rules must apply in every case.
A) The ring system must be planar.
B) The system must be monocyclic.
C) There must be (4n + 2) electrons.
D) The Hückel number of electrons must be completely delocalized.
E) None.All of these rules must apply in every case.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following statements regarding the cyclopropenyl anion is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many different dichloronitrobenzenes are possible?
A) 8
B) 7
C) 6
D) 5
E) 4
A) 8
B) 7
C) 6
D) 5
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following is true of benzene?
A) Benzene tends to undergo addition rather than substitution reactions.
B) All of the hydrogen atoms of benzene are equivalent.
C) The carbon-carbon bonds of benzene are alternately short and long around the ring.
D) The benzene ring is a distorted hexagon.
E) Benzene has the stability expected for cyclohexatriene.
A) Benzene tends to undergo addition rather than substitution reactions.
B) All of the hydrogen atoms of benzene are equivalent.
C) The carbon-carbon bonds of benzene are alternately short and long around the ring.
D) The benzene ring is a distorted hexagon.
E) Benzene has the stability expected for cyclohexatriene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
26
In which of the following compounds would the shortest carbon-carbon bond(s)be found?
A) Toluene
B) 2-Ethylcyclopentene
C) 4-Methyl-1,3-cyclohexadiene
D) 3-Methyl-1-hexyne
E) 3-Methyl-1,3-hexadiene
A) Toluene
B) 2-Ethylcyclopentene
C) 4-Methyl-1,3-cyclohexadiene
D) 3-Methyl-1-hexyne
E) 3-Methyl-1,3-hexadiene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
27
Why would 1,3-cyclohexadiene undergo dehydrogenation readily?
A) It is easily reduced.
B) Hydrogen is a small molecule.
C) 1,3-Cyclohexadiene has no resonance energy.
D) It would gain considerable stability by becoming benzene.
E) It would not undergo dehydrogenation.
A) It is easily reduced.
B) Hydrogen is a small molecule.
C) 1,3-Cyclohexadiene has no resonance energy.
D) It would gain considerable stability by becoming benzene.
E) It would not undergo dehydrogenation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements regarding the cycloheptatrienyl anion is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
29
In the molecular orbital model of benzene,how many pi-electrons are delocalized about the ring?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following is NOT true of benzene?
A) Benzene tends to undergo substitution rather than addition reactions,even though it has a high index of hydrogen deficiency.
B) All of the hydrogen atoms of benzene are equivalent.
C) The carbon-carbon bonds of benzene are alternately short and long around the ring.
D) Only one o-dichlorobenzene has ever been found.
E) Benzene is more stable than the hypothetical compound 1,3,5-cyclohexatriene.
A) Benzene tends to undergo substitution rather than addition reactions,even though it has a high index of hydrogen deficiency.
B) All of the hydrogen atoms of benzene are equivalent.
C) The carbon-carbon bonds of benzene are alternately short and long around the ring.
D) Only one o-dichlorobenzene has ever been found.
E) Benzene is more stable than the hypothetical compound 1,3,5-cyclohexatriene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
31
In the molecular orbital model of benzene,how many pi-electrons are in bonding molecular orbitals?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
32
We now know that the two Kekule structures for benzene are related in the following way:
A) They are each equally correct as a structure for benzene.
B) Benzene is sometimes one structure and sometimes the other.
C) The two structures are in a state of rapid equilibrium.
D) Neither of the two structures adequately describes benzene;benzene is a resonance hybrid of the two.
E) None of the above
A) They are each equally correct as a structure for benzene.
B) Benzene is sometimes one structure and sometimes the other.
C) The two structures are in a state of rapid equilibrium.
D) Neither of the two structures adequately describes benzene;benzene is a resonance hybrid of the two.
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements regarding the cyclopentadienyl radical is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Huckel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Huckel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
34
The carbon-carbon bonds in benzene are:
A) of equal length and are shorter than the double bond of ethene.
B) of equal length and are intermediate between a double bond and a single bond.
C) of unequal length and are alternately short and long around the ring.
D) due only to p-orbital overlap.
E) of equal length and intermediate between the carbon-carbon bond lengths in ethene and ethyne.
A) of equal length and are shorter than the double bond of ethene.
B) of equal length and are intermediate between a double bond and a single bond.
C) of unequal length and are alternately short and long around the ring.
D) due only to p-orbital overlap.
E) of equal length and intermediate between the carbon-carbon bond lengths in ethene and ethyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following statements regarding the cycloheptatrienyl radical is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
36
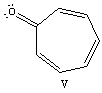
Recalling that benzene has a resonance energy of 152 kJ mol-1 and naphthalene has a resonance energy of 255 kJ mol-1,predict the positions which would be occupied by bromine when phenanthrene (below)undergoes addition of Br2. 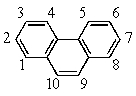
A) 1,2
B) 1,4
C) 3,4
D) 7,8
E) 9,10

A) 1,2
B) 1,4
C) 3,4
D) 7,8
E) 9,10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following statements regarding the cyclopropenyl radical is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
38
How many different dihydroxybromobenzenes are possible?
A) 8
B) 7
C) 6
D) 5
E) 4
A) 8
B) 7
C) 6
D) 5
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
39
Consider the molecular orbital model of benzene.In the ground state how many molecular orbitals are filled with electrons?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following statements regarding the cyclopentadienyl cation is correct?
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.
A) It is aromatic.
B) It is not aromatic.
C) It obeys Hückel's rule.
D) It undergoes reactions characteristic of benzene.
E) It has a closed shell of 6 pi-electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following would you expect to be aromatic? 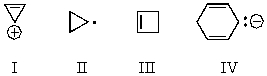
A) I
B) II
C) III
D) IV
E) All of these

A) I
B) II
C) III
D) IV
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
42
On the basis of molecular orbital theory and Hückel's rule,which molecules and/or ions should be non-aromatic? 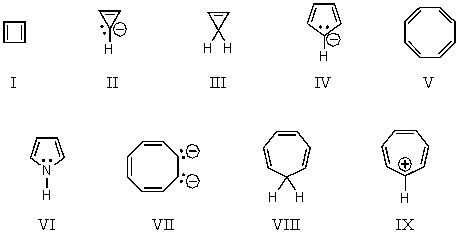
A) I and V
B) I and II
C) III and VIII
D) IV,VII and IX
E) IV,VI,VII and IX

A) I and V
B) I and II
C) III and VIII
D) IV,VII and IX
E) IV,VI,VII and IX

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
43
In the molecular orbital model of benzene,how many -bonding molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
44
In the molecular orbital model of benzene,the six p-orbitals combine to form how many molecular orbitals?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
45
On the basis of molecular orbital theory and Hückel's rule,which of these compounds should be aromatic? 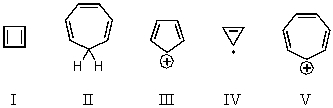
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many equivalent resonance structures can be written for the cycloheptatrienyl cation?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
47
On the basis of molecular orbital theory and Hückel's rule,which molecules and/or ions should be aromatic? 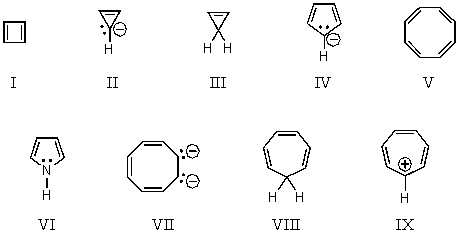
A) I and V
B) III and VIII
C) IV,VII and IX
D) IV,VI,VII and IX
E) All of the structures,I-IX

A) I and V
B) III and VIII
C) IV,VII and IX
D) IV,VI,VII and IX
E) All of the structures,I-IX

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following would you expect to be aromatic? 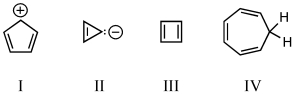

A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
49
How many equivalent resonance structures can be written for the cyclopentadienyl anion?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of these is an aromatic molecule? 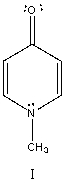
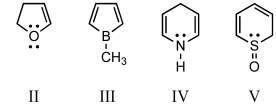
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
51
Cyclopentadiene is unusually acidic for a hydrocarbon.An explanation for this is the following statement.
A) The carbon atoms of cyclopentadiene are all sp2-hybridized.
B) Cyclopentadiene is aromatic.
C) Removal of a proton from cyclopentadiene yields an aromatic anion.
D) Removal of a hydrogen atom from cyclopentadiene yields a highly stable free radical.
E) Removal of a hydride ion from cyclopentadiene produces an aromatic cation.
A) The carbon atoms of cyclopentadiene are all sp2-hybridized.
B) Cyclopentadiene is aromatic.
C) Removal of a proton from cyclopentadiene yields an aromatic anion.
D) Removal of a hydrogen atom from cyclopentadiene yields a highly stable free radical.
E) Removal of a hydride ion from cyclopentadiene produces an aromatic cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following would you expect to be aromatic? 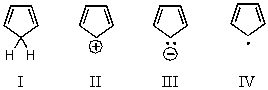
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
53
On the basis of molecular orbital theory and Hückel's rule,which of these compounds should be aromatic? 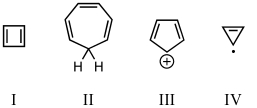
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
54
On the basis of molecular orbital theory and Hückel's rule,which molecules and/or ions should be antiaromatic? 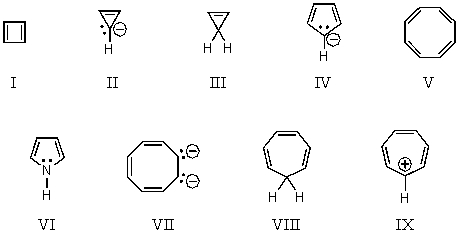
A) I and V
B) I and II
C) III and VIII
D) IV,VII and IX
E) IV,VI,VII and IX

A) I and V
B) I and II
C) III and VIII
D) IV,VII and IX
E) IV,VI,VII and IX

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following would you expect to be aromatic? 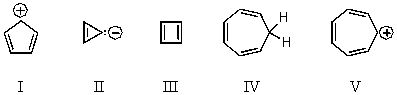
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following would you expect to be aromatic? 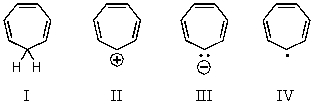
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
57
Application of the polygon-and-circle technique reveals that single electrons occupy each of the two nonbonding orbitals in the molecular orbital diagram of:
A) Cyclobutadiene
B) Benzene
C) Cyclopropenyl cation
D) Cyclopentadienyl anion
E) Cycloheptatrienyl cation
A) Cyclobutadiene
B) Benzene
C) Cyclopropenyl cation
D) Cyclopentadienyl anion
E) Cycloheptatrienyl cation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following structures would be aromatic? 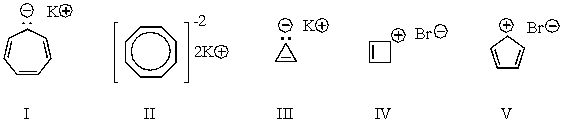
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of these is an aromatic molecule? 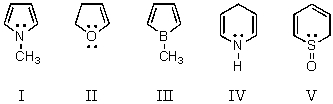
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following statements about cyclooctatetraene is NOT true?
A) The compound rapidly decolorizes Br2/CCl4 solutions.
B) The compound rapidly decolorizes aqueous solutions of KMnO4.
C) The compound readily adds hydrogen.
D) The compound is nonplanar.
E) The compound is comparable to benzene in stability.
A) The compound rapidly decolorizes Br2/CCl4 solutions.
B) The compound rapidly decolorizes aqueous solutions of KMnO4.
C) The compound readily adds hydrogen.
D) The compound is nonplanar.
E) The compound is comparable to benzene in stability.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
61
In the molecular orbital model of the cyclopentadienyl cation,how many non-bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
62
In the molecular orbital model of cyclobutadiene,how many pairs of degenerate -antibonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
63
In the molecular orbital model of benzene,how many -antibonding molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
64
In the molecular orbital model of cyclobutadiene,how many -bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
65
In the molecular orbital model of cyclobutadiene,how many non-bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
66
In the molecular orbital model of the cyclopentadienyl cation,how many pairs of degenerate -antibonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
67
In the molecular orbital model of benzene,how many pairs of degenerate -bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
68
In the molecular orbital model of the cyclopentadienyl cation,how many pairs of degenerate -molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
69
Consider the molecular orbital model of cyclopentadienyl anion.In the ground state how many molecular orbitals are filled with electrons?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
70
In the molecular orbital model of cyclobutadiene,how many -antibonding molecular orbitals are there?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following would you expect to be antiaromatic? 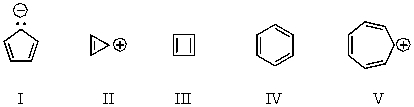
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
72
In the molecular orbital model of cyclobutadiene,how many pairs of degenerate -molecular orbitals are there?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
73
In the molecular orbital model of benzene,how many pairs of degenerate -antibonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
74
In the molecular orbital model of the cyclopentadienyl cation,how many pairs of degenerate -bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
75
In the molecular orbital model of the cyclopentadienyl cation,how many -bonding molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
76
In the molecular orbital model of benzene,how many pairs of degenerate -molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
77
In the molecular orbital model of the cyclobutadiene,how many pairs of degenerate -bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
78
In the molecular orbital model of the cyclopentadienyl cation,how many -antibonding molecular orbitals are there?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
79
In the molecular orbital model of benzene,how many non-bonding molecular orbitals are there?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck
80
Of the following C-10 compounds,which is expected to possess the greatest resonance (delocalization)energy? 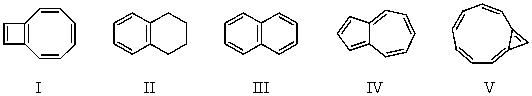
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 151 في هذه المجموعة.
فتح الحزمة
k this deck








