Deck 4: Energy and Enzymes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/80
العب
ملء الشاشة (f)
Deck 4: Energy and Enzymes
1
Which statement best describes the three types of systems?
A)An isolated system does not exchange energy or matter with its surroundings; a closed system exchanges only energy, but not matter with its surroundings; an open system exchanges both energy and matter with its surroundings.
B)An isolated system does not exchange energy or matter with its surroundings; a closed system exchanges only matter, but not energy with its surroundings; an open system exchanges both energy and matter with its surroundings.
C)An isolated system exchanges energy with its surroundings; a closed system exchanges only matter, but not energy with its surroundings; an open system exchanges both energy and matter with its surroundings.
D)An isolated system exchanges energy with its surroundings; a closed system exchanges only energy, but not matter with its surroundings; an open system exchanges matter, but not energy with its surroundings.
A)An isolated system does not exchange energy or matter with its surroundings; a closed system exchanges only energy, but not matter with its surroundings; an open system exchanges both energy and matter with its surroundings.
B)An isolated system does not exchange energy or matter with its surroundings; a closed system exchanges only matter, but not energy with its surroundings; an open system exchanges both energy and matter with its surroundings.
C)An isolated system exchanges energy with its surroundings; a closed system exchanges only matter, but not energy with its surroundings; an open system exchanges both energy and matter with its surroundings.
D)An isolated system exchanges energy with its surroundings; a closed system exchanges only energy, but not matter with its surroundings; an open system exchanges matter, but not energy with its surroundings.
A
2
Which of the following occurs when there are more reactants than products for a reaction?
A)The reaction is pushed toward the reactants by the low concentration of products.
B)The reaction is pulled toward generating more reactants.
C)The greater concentration of reactants pushes the reaction forward, toward generating more products.
D)The reaction is pulled in the forward direction by the high concentration of products.
A)The reaction is pushed toward the reactants by the low concentration of products.
B)The reaction is pulled toward generating more reactants.
C)The greater concentration of reactants pushes the reaction forward, toward generating more products.
D)The reaction is pulled in the forward direction by the high concentration of products.
C
3
Which of the following is an example of a closed system?
A)one that exchanges matter or energy with its surroundings
B)one that does not exchange matter or energy with its surroundings
C)one that exchanges only matter with its surroundings
D)one that exchanges only energy with its surroundings
A)one that exchanges matter or energy with its surroundings
B)one that does not exchange matter or energy with its surroundings
C)one that exchanges only matter with its surroundings
D)one that exchanges only energy with its surroundings
D
4
Which of the following is NOT a form of energy?
A)heat
B)diffusion
C)light
D)sound
A)heat
B)diffusion
C)light
D)sound

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following best describes why thinking of entropy as disorder is problematic?
A)This idea can be applied scientifically in a precise way, and entropy is not governed by order.
B)This idea can be applied scientifically in a precise way, and entropy is governed by order.
C)This idea cannot be applied scientifically in a precise way, and entropy is governed by order.
D)This idea cannot be applied scientifically in a precise way, and entropy is not governed by order.
A)This idea can be applied scientifically in a precise way, and entropy is not governed by order.
B)This idea can be applied scientifically in a precise way, and entropy is governed by order.
C)This idea cannot be applied scientifically in a precise way, and entropy is governed by order.
D)This idea cannot be applied scientifically in a precise way, and entropy is not governed by order.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
6
What types of energy are utilized by a child swinging on a swing?
A)chemical energy only, because it is the child's metabolism that powers the muscles that make the swing move
B)kinetic, potential, and chemical energy: the child powers the swing with chemical energy in the muscle cells, and the swing moves like a pendulum with changing ratios of kinetic and potential energy
C)kinetic and potential energy only, but in constantly changing ratios: when changing direction, the energy is pure potential energy, but at the bottom of the arc, the energy is pure kinetic energy
D)kinetic energy only, because the child is in constant motion
A)chemical energy only, because it is the child's metabolism that powers the muscles that make the swing move
B)kinetic, potential, and chemical energy: the child powers the swing with chemical energy in the muscle cells, and the swing moves like a pendulum with changing ratios of kinetic and potential energy
C)kinetic and potential energy only, but in constantly changing ratios: when changing direction, the energy is pure potential energy, but at the bottom of the arc, the energy is pure kinetic energy
D)kinetic energy only, because the child is in constant motion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
7
Why do reversible reactions in a cell rarely reach equilibrium?
A)because the products are generally reactants in other reactions and are thus immediately used
B)because most reactions in a cell are not reversible, thus allowing the cell to devote additional resources to regulating the few reversible reactions that do occur
C)because cells have no way of measuring the relative ratios of reactants and products
D)because a cell at equilibrium is dead
A)because the products are generally reactants in other reactions and are thus immediately used
B)because most reactions in a cell are not reversible, thus allowing the cell to devote additional resources to regulating the few reversible reactions that do occur
C)because cells have no way of measuring the relative ratios of reactants and products
D)because a cell at equilibrium is dead

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following can be said to occur during every energy transformation?
A)A change occurs in the free energy of the universe.
B)The entropy of the universe increases.
C)A change occurs in the total energy of the universe.
D)The entropy of the universe decreases.
A)A change occurs in the free energy of the universe.
B)The entropy of the universe increases.
C)A change occurs in the total energy of the universe.
D)The entropy of the universe decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following is a closed system?
A)a single-celled organism
B)a human
C)a gas-powered automobile
D)the Earth
A)a single-celled organism
B)a human
C)a gas-powered automobile
D)the Earth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following best describes why bricks from a truck fall all over during a traffic accident?
A)The bricks have reached an equilibrium state.
B)The bricks have reached a minimum entropy state.
C)The bricks have reached a maximum free energy state.
D)The bricks have reached a minimum free energy state.
A)The bricks have reached an equilibrium state.
B)The bricks have reached a minimum entropy state.
C)The bricks have reached a maximum free energy state.
D)The bricks have reached a minimum free energy state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following best describes an ocean as an open system?
A)It does not exchange matter with its surroundings.
B)It absorbs but not releases energy.
C)It absorbs and releases energy.
D)It exchanges energy but not matter with its surroundings.
A)It does not exchange matter with its surroundings.
B)It absorbs but not releases energy.
C)It absorbs and releases energy.
D)It exchanges energy but not matter with its surroundings.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following is a correct pair based on their shared characteristics?
A)Earth and a closed system
B)the universe and an open system
C)an ocean and a closed system
D)an ocean and an isolated system
A)Earth and a closed system
B)the universe and an open system
C)an ocean and a closed system
D)an ocean and an isolated system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following best illustrates the first law of thermodynamics by Niagara Falls?
A)Water at the top of the falls has high kinetic energy; as the water moves over the waterfall, its kinetic energy is converted into potential energy; when the water reaches the bottom of the waterfall, its potential energy is transformed into other types of energy: heat, sound, and mechanical energy.
B)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is transformed into other types of energy: heat, sound, and mechanical energy.
C)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is transformed into potential energy again.
D)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is lost.
A)Water at the top of the falls has high kinetic energy; as the water moves over the waterfall, its kinetic energy is converted into potential energy; when the water reaches the bottom of the waterfall, its potential energy is transformed into other types of energy: heat, sound, and mechanical energy.
B)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is transformed into other types of energy: heat, sound, and mechanical energy.
C)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is transformed into potential energy again.
D)Water at the top of the falls has high potential energy; as the water moves over the waterfall, its potential energy is converted into kinetic energy; when the water reaches the bottom of the waterfall, its kinetic energy is lost.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which reaction is likely to have more products than reactants when the reaction reaches equilibrium?
A)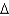 G = -25 kcal/mol
G = -25 kcal/mol
B)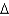 G = -50 kcal/mol
G = -50 kcal/mol
C)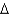 G = -75 kcal/mol
G = -75 kcal/mol
D)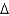 G = -100 kcal/mol
G = -100 kcal/mol
A)
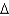 G = -25 kcal/mol
G = -25 kcal/molB)
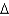 G = -50 kcal/mol
G = -50 kcal/molC)
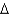 G = -75 kcal/mol
G = -75 kcal/molD)
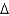 G = -100 kcal/mol
G = -100 kcal/mol
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
15
Suppose that an earthquake hits. Which of the following would be most likely to characterize your books being scattered on the floor all over your room?
A)The books now have less entropy.
B)The books now have more entropy.
C)The books have the same entropy.
D)The books have no entropy.
A)The books now have less entropy.
B)The books now have more entropy.
C)The books have the same entropy.
D)The books have no entropy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following best describes a dead cell as a system?
A)It is a closed system that exchanges energy with its surroundings.
B)It is an open system that exchanges energy with its surroundings.
C)It is an isolated system that does not exchange energy or matter with its surroundings.
D)It is a closed system that does not exchange energy or matter with its surroundings.
A)It is a closed system that exchanges energy with its surroundings.
B)It is an open system that exchanges energy with its surroundings.
C)It is an isolated system that does not exchange energy or matter with its surroundings.
D)It is a closed system that does not exchange energy or matter with its surroundings.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which statement best describes the first law of thermodynamics?
A)Matter can be created and destroyed.
B)Matter only changes forms.
C)Energy only changes forms.
D)Energy can be created and destroyed.
A)Matter can be created and destroyed.
B)Matter only changes forms.
C)Energy only changes forms.
D)Energy can be created and destroyed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following best explains why machines can never be 100% efficient?
A)the tendency of energy not to spread out
B)the tendency of energy to spread out
C)the tendency of energy to be kept
D)the tendency of energy to be lost
A)the tendency of energy not to spread out
B)the tendency of energy to spread out
C)the tendency of energy to be kept
D)the tendency of energy to be lost

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following is a correct pair based on their shared characteristics?
A)the universe and a closed system
B)the universe and an open system
C)an ocean and an open system
D)an ocean and a closed system
A)the universe and a closed system
B)the universe and an open system
C)an ocean and an open system
D)an ocean and a closed system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following is an exergonic reaction?
A)folding laundry
B)synthesizing a protein
C)burning wood for a campfire
D)building a tower out of blocks
A)folding laundry
B)synthesizing a protein
C)burning wood for a campfire
D)building a tower out of blocks

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following best describes how enzymes function?
A)by slowing the rate of some reactions and increasing the rate of other reactions
B)by increasing the rate of a reaction
C)by adding additional reactants to the system
D)by changing the ?G of the reaction
A)by slowing the rate of some reactions and increasing the rate of other reactions
B)by increasing the rate of a reaction
C)by adding additional reactants to the system
D)by changing the ?G of the reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which phrase best describes how endergonic reactions are coupled within a cell?
A)Endergonic reactions are coupled to endergonic reactions to produce ATP.
B)Endergonic reactions are coupled to exergonic reactions to produce ATP.
C)Endergonic reactions are coupled to exergonic reactions to produce AMP.
D)Endergonic reactions are coupled to endergonic reactions to produce AMP.
A)Endergonic reactions are coupled to endergonic reactions to produce ATP.
B)Endergonic reactions are coupled to exergonic reactions to produce ATP.
C)Endergonic reactions are coupled to exergonic reactions to produce AMP.
D)Endergonic reactions are coupled to endergonic reactions to produce AMP.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following occurs when a reaction reaches equilibrium?
A)The rates of the forward and reverse reactions are equal.
B)The chemical reactions cease.
C)Entropy is no longer in the system.
D)The concentration of reactants equals the concentration of products.
A)The rates of the forward and reverse reactions are equal.
B)The chemical reactions cease.
C)Entropy is no longer in the system.
D)The concentration of reactants equals the concentration of products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
24
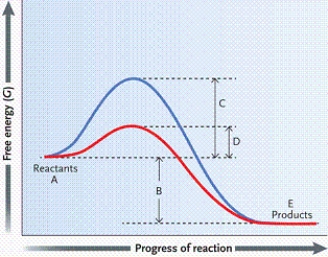
Which letter on the graph indicates the activation energy when no enzyme is present?
A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
25
When ATP is split into ADP and Pi, what happens to the energy that gets released?
A)The energy is transferred to the target molecule via the transfer of Pi.
B)The energy is directly transferred to the target molecule by an unknown mechanism.
C)The two remaining phosphates acquire the energy that was present in the linkage of three phosphates.
D)The energy dissipates in the form of heat.
A)The energy is transferred to the target molecule via the transfer of Pi.
B)The energy is directly transferred to the target molecule by an unknown mechanism.
C)The two remaining phosphates acquire the energy that was present in the linkage of three phosphates.
D)The energy dissipates in the form of heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which type of metabolic pathway involves building biomass?
A)catabolic
B)parabolic
C)irreversible
D)anabolic
A)catabolic
B)parabolic
C)irreversible
D)anabolic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
27
Enzymes work with at least three mechanisms. Which of the following is NOT a mechanism by which enzymes function?
A)by putting reactants in close proximity to each other
B)by altering the free energy ( G) of the reaction
G) of the reaction
C)by orienting the reactants so they are positioned to favour the transition state
D)by altering the immediate environment of the reactants to promote reactant interactions
A)by putting reactants in close proximity to each other
B)by altering the free energy (
 G) of the reaction
G) of the reactionC)by orienting the reactants so they are positioned to favour the transition state
D)by altering the immediate environment of the reactants to promote reactant interactions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which sentence best describes the process of competitive inhibition?
A)The inhibitor binds to and directly blocks the active site of the enzyme.
B)The products of the reaction block the active site of the enzyme.
C)The substrate and cofactors compete for the active site.
D)The inhibitor binds to a site other than the active site of the enzyme and blocks enzyme activity indirectly.
A)The inhibitor binds to and directly blocks the active site of the enzyme.
B)The products of the reaction block the active site of the enzyme.
C)The substrate and cofactors compete for the active site.
D)The inhibitor binds to a site other than the active site of the enzyme and blocks enzyme activity indirectly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
29

Which letter on the graph indicates the activation energy when an enzyme is present?
A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
30
Suppose you conduct an experiment in the laboratory in which you add increasing amounts of substrate to a solution containing an enzyme and a pH buffer. You incubate the container at the optimal temperature for the enzyme. Each time you add more substrate, you measure the rate of the reaction. Also suppose that you graph the results such that the x-axis shows the substrate concentration and the y-axis shows the resulting reaction rate. What will you find over time?
A)The rate of the reaction will increase rapidly, taper off, and plateau.
B)The resulting graph will be a perfect bell curve.
C)The rate of the reaction will increase slowly, plateau, and then drop sharply back to zero.
D)The rate of the reaction will proceed with a slope of 1 and continue in a linear fashion indefinitely or until you run out of reactants.
A)The rate of the reaction will increase rapidly, taper off, and plateau.
B)The resulting graph will be a perfect bell curve.
C)The rate of the reaction will increase slowly, plateau, and then drop sharply back to zero.
D)The rate of the reaction will proceed with a slope of 1 and continue in a linear fashion indefinitely or until you run out of reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
31

Which letter on the graph indicates the free energy of the reaction?
A)A
B)B
C)C
D)D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which sentence best describes the situation that occurs when an enzyme and its substrate interact?
A)Just after substrate binding, the enzyme changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the substrate.
B)Just before substrate binding, the enzyme changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the substrate.
C)Just before substrate binding, the substrate changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the enzyme.
D)Just after substrate binding, the substrate changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the enzyme.
A)Just after substrate binding, the enzyme changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the substrate.
B)Just before substrate binding, the enzyme changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the substrate.
C)Just before substrate binding, the substrate changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the enzyme.
D)Just after substrate binding, the substrate changes its shape (conformation) so that the active site becomes even more precise in its ability to bind the enzyme.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
33

Which letter on the graph indicates the free energy of the reactants?
A)A
B)B
C)D
D)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
34
What does it mean when an enzyme is saturated?
A)It means the enzymes need more reactants.
B)It means the enzymes have sufficient reactants available for optimal activity.
C)It means the enzymes cannot continue to catalyze the reaction.
D)It means the reaction is at equilibrium.
A)It means the enzymes need more reactants.
B)It means the enzymes have sufficient reactants available for optimal activity.
C)It means the enzymes cannot continue to catalyze the reaction.
D)It means the reaction is at equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the difference between cofactors and coenzymes?
A)Cofactors tend to be complex molecules, but coenzymes are generally ions.
B)Cofactors are not necessary, but coenzymes are necessary.
C)Cofactors can be inorganic or organic, but coenzymes are always inorganic.
D)Cofactors can be inorganic or organic, but coenzymes are just another name for organic cofactors.
A)Cofactors tend to be complex molecules, but coenzymes are generally ions.
B)Cofactors are not necessary, but coenzymes are necessary.
C)Cofactors can be inorganic or organic, but coenzymes are always inorganic.
D)Cofactors can be inorganic or organic, but coenzymes are just another name for organic cofactors.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
36
What does it mean that cells are open systems?
A)They take out energy and matter and maintain ordered state.
B)They bring in energy and matter and maintain disordered state.
C)They take out energy and matter and maintain ordered state.
D)They bring in energy and matter and maintain ordered state.
A)They take out energy and matter and maintain ordered state.
B)They bring in energy and matter and maintain disordered state.
C)They take out energy and matter and maintain ordered state.
D)They bring in energy and matter and maintain ordered state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following describes a main mechanism of enzymes?
A)removing reactants from a solution in a set ratio that enhances the chances of the remaining individual reactants interacting with each other
B)forcing the reactants into an altered environment, which in turn creates a change in the free energy of the reactants relative to the products
C)increasing the probability that the reactants will come into close proximity to each other in the proper orientation for forming the transition state molecule
D)altering the equilibrium point of a particular reaction to favour the formation of products
A)removing reactants from a solution in a set ratio that enhances the chances of the remaining individual reactants interacting with each other
B)forcing the reactants into an altered environment, which in turn creates a change in the free energy of the reactants relative to the products
C)increasing the probability that the reactants will come into close proximity to each other in the proper orientation for forming the transition state molecule
D)altering the equilibrium point of a particular reaction to favour the formation of products

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
38
Suppose you decide to alter the rate of a reaction. Which of the following would tend to reverse the enzyme-catalyzed reaction?
A)adding more reactants
B)adding more enzyme
C)mechanically stirring the contents of a beaker of reactants
D)adding more product
A)adding more reactants
B)adding more enzyme
C)mechanically stirring the contents of a beaker of reactants
D)adding more product

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which sentence best describes the process of noncompetitive feedback inhibition?
A)The inhibitor binds to the active site and directly blocks the active site of the enzyme.
B)The products of the reaction at the end of the pathway bind to a site other than the active site of an enzyme at or near the beginning of the pathway, and block enzyme activity indirectly.
C)The products of the reaction at the end of the pathway block the active site of the enzyme, inhibiting it directly.
D)The substrate and cofactors compete for the active site of an enzyme and block enzyme activity directly.
A)The inhibitor binds to the active site and directly blocks the active site of the enzyme.
B)The products of the reaction at the end of the pathway bind to a site other than the active site of an enzyme at or near the beginning of the pathway, and block enzyme activity indirectly.
C)The products of the reaction at the end of the pathway block the active site of the enzyme, inhibiting it directly.
D)The substrate and cofactors compete for the active site of an enzyme and block enzyme activity directly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
40

Which letter on the graph indicates the free energy of the products?
A)B
B)C
C)D
D)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
41
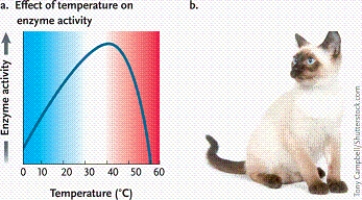 Photomicrograph by Dr. Conly L. Rieder, East Greenbush, New York
Photomicrograph by Dr. Conly L. Rieder, East Greenbush, New YorkWhich of the following hypotheses do you believe is best supported by the data in the graph?
A)The enzyme will completely denature at 38ºC.
B)The enzyme will probably be inactive at a pH below 4.5.
C)The enzyme has a cofactor.
D)The enzyme's activity will drop at temperatures above 40ºC, and activity will likely be eliminated by 55ºC.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
42
 Photomicrograph by Dr. Conly L. Rieder, East Greenbush, New York
Photomicrograph by Dr. Conly L. Rieder, East Greenbush, New YorkIn the graph, why does the curve sharply drop after approximately 45°C instead of mirroring the slope of the line going from 0 to 40°C?
A)The kinetic energy of the reactants is so great that it destabilizes the enzyme and diminishes the enzyme's activity.
B)This is true of all catalysts and is not due to any special features of enzymes.
C)The kinetic energy of the reactants is lower than that of the products, thus forcing a change in enzyme activity.
D)The enzyme begins to denature above a certain temperature, thus eliminating all catalytic activity of the protein.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
43
What does it mean when an enzyme has an allosteric activator?
A)The product of the enzyme, or another downstream product, will bind to the enzyme at a site other than the active site and inhibit enzyme activity.
B)The product of the enzyme, or another downstream product, will bind to the enzyme at the active site and stimulate enzyme activity.
C)The product of the enzyme, or another downstream product, will bind to the enzyme at a site other than the active site and stimulate enzyme activity.
D)The product of the enzyme, or another downstream product, will bind to the enzyme at the active site and inhibit enzyme activity.
A)The product of the enzyme, or another downstream product, will bind to the enzyme at a site other than the active site and inhibit enzyme activity.
B)The product of the enzyme, or another downstream product, will bind to the enzyme at the active site and stimulate enzyme activity.
C)The product of the enzyme, or another downstream product, will bind to the enzyme at a site other than the active site and stimulate enzyme activity.
D)The product of the enzyme, or another downstream product, will bind to the enzyme at the active site and inhibit enzyme activity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
44
Enzyme activity is increased by falling temperatures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
45
AMP is the primary energy and phosphate source in coupled reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
46
In the diagram, suppose the enzyme catalyzing the intermediate B to intermediate C reaction is inhibited. Which compound is most likely to accumulate?
A)intermediate A
B)intermediate B
C)intermediate C
D)intermediate D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
47
Enzymes alter the equilibrium point of a reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
48
All true enzymes are proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
49
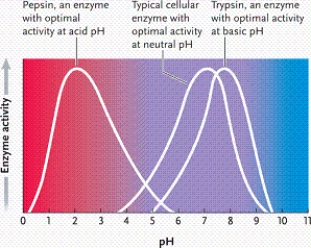
Refer to the graph. Which phrase best describes what it represents?
A)enzyme activity as a function of pH in a fairly neutral environment
B)enzyme activity as a function of pH in a strongly acidic environment
C)enzyme activity as a function of temperature
D)enzyme activity as a function of pH in a strongly basic environment

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
50
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
metabolic pathway
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
metabolic pathway

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
51
Explain three ways in which other molecules regulate enzymes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
52
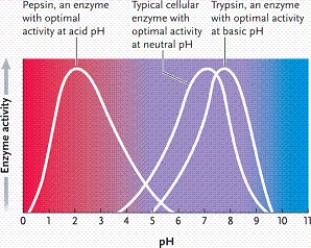
Suppose all three enzymes represented in the graph catalyze the same reaction, but conditions require you to use a pH of 7. Which is the best enzyme to use?
A)enzyme 3
B)enzyme 2
C)either enzyme 1 or 2
D)enzyme 1
A)enzyme 3
B)enzyme 2
C)either enzyme 1 or 2
D)enzyme 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
53
At equilibrium, the concentration of the reactants equals the concentration of the products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
54
Reactions that reach an equilibrium point are reversible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
55

According to the graph, what is the optimal pH for enzyme 1?
A)2
B)3
C)4
D)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
56

According to the graph, what is the optimal pH for enzyme 2?
A)6
B)7
C)8
D)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
57
Explain how a cell can use catabolic reactions to drive anabolic reactions, despite energy loss in the form of entropy and heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
58
Explain how temperature can affect enzyme activity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
59
Enzymes do not change the ?G of a reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
60
Activation energy is not required for nonspontaneous reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
61
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
folding laundry
a.endergonic
b.exergonic
c.equilibrium
folding laundry

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
62
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G is positive
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G is positive

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
63
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
protein synthesis
a.endergonic
b.exergonic
c.equilibrium
protein synthesis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
64
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
activation energy
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
activation energy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
65
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
the rate of synthesis equals the rate of degradation
a.endergonic
b.exergonic
c.equilibrium
the rate of synthesis equals the rate of degradation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
66
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
coupled reaction
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
coupled reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
67
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
equilibrium point
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
equilibrium point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
68
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
phosphorylation
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
phosphorylation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
69
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G is negative
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G is negative

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
70
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
ATP
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
ATP

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
71
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a beaker of water sitting on a bench
a.endergonic
b.exergonic
c.equilibrium
a beaker of water sitting on a bench

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
72
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
digestion of a candy bar
a.endergonic
b.exergonic
c.equilibrium
digestion of a candy bar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
73
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
transition state
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
transition state

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
74
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G = 0
a.endergonic
b.exergonic
c.equilibrium
a reaction where ?G = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
75
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
active site
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
active site

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
76
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a toddler dumping boxes of toys
a.endergonic
b.exergonic
c.equilibrium
a toddler dumping boxes of toys

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
77
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
substrate
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
substrate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
78
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
allosteric regulation
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
allosteric regulation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
79
Match each definition with the corresponding term.
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
catalyst
a.primary coupling agent in cellular reactions
b.addition of a phosphate group to a target molecule
c.product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
d.linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
e.series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
f.substance that facilitates a chemical reaction without itself being consumed by the reaction
g.energy needed to start a reaction, be it endergonic or exergonic
h.portion of the enzyme that binds to a reactant or reactants
i.state in which the rate of the forward reaction equals the rate of the reverse reaction
j.intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
k.reactant molecule that binds to an enzyme
catalyst

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck
80
For each situation, choose the most appropriate term.
a.endergonic
b.exergonic
c.equilibrium
a dead cell
a.endergonic
b.exergonic
c.equilibrium
a dead cell

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 80 في هذه المجموعة.
فتح الحزمة
k this deck








