Deck 20: Amines
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/207
العب
ملء الشاشة (f)
Deck 20: Amines
1
The correct structure of (S)-bicyclo[2.2.1]heptan-2-amine is? ![<strong>The correct structure of (S)-bicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8520_9180_4f89eafc94c1_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure of (S)-bicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8520_9180_4f89eafc94c1_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
III
2
The correct structure of bicyclo[1.1.0]butan-1-amine is? ![<strong>The correct structure of bicyclo[1.1.0]butan-1-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_851f_9180_33bd12d4d217_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure of bicyclo[1.1.0]butan-1-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_851f_9180_33bd12d4d217_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
II
3
The correct structure of (S)-N-methylbicyclo[2.2.1]heptan-2-amine is? ![<strong>The correct structure of (S)-N-methylbicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8521_9180_f929aa8b6831_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure of (S)-N-methylbicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8521_9180_f929aa8b6831_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
III
4
Which is a correct common name for the following substance? 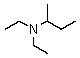
A)Ethylethylisobutylamine
B)Diethylisobutylamine
C)sec-Butyldiethylamine
D)Ethylethyl-sec-butylamine
E)2-Diethylaminobutane

A)Ethylethylisobutylamine
B)Diethylisobutylamine
C)sec-Butyldiethylamine
D)Ethylethyl-sec-butylamine
E)2-Diethylaminobutane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
5
What type of amine is piperidine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
6
What type of amine is quinoline?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
7
What type of amine is pyrrole?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
8
The correct structure of bicyclo[1.1.0]butan-2-amine is? ![<strong>The correct structure of bicyclo[1.1.0]butan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_5e0e_9180_c71f5656d722_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure of bicyclo[1.1.0]butan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_5e0e_9180_c71f5656d722_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which is a correct IUPAC name for the following substance? 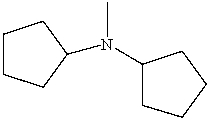
A)N-Cyclopentyl-N-methylcyclopentanamine
B)N-Cyclohexyl-N-methylcyclopentanamine
C)N-Cyclopentyl-N-methylcyclohexanamine
D)Dicyclopentylmethylamine
E)N-Pentyl-N-methylpentanamine

A)N-Cyclopentyl-N-methylcyclopentanamine
B)N-Cyclohexyl-N-methylcyclopentanamine
C)N-Cyclopentyl-N-methylcyclohexanamine
D)Dicyclopentylmethylamine
E)N-Pentyl-N-methylpentanamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
10
What type of amine is pyridine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of these is properly termed a "quaternary ammonium salt"?
A)(CH3)3CCH2CH2NH3+ Cl-
B)(CH3CH2CH(CH3)CH2)2NH2+ Cl-
C)(CH3CH2CH2)3NH+ Cl-
D)(CH3CH2CH2)4N+ Cl-
E)None of these choices.
A)(CH3)3CCH2CH2NH3+ Cl-
B)(CH3CH2CH(CH3)CH2)2NH2+ Cl-
C)(CH3CH2CH2)3NH+ Cl-
D)(CH3CH2CH2)4N+ Cl-
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
12
The aromatic amine purine contains how many nitrogen atoms?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which is a correct IUPAC name for the following substance? 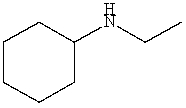
A)N-Ethylhexanamine
B)N-Ethylcyclohexanamine
C)N-Cyclohexylethanamine
D)N-Ethylcyclopentanamine
E)N-Ethylaniline

A)N-Ethylhexanamine
B)N-Ethylcyclohexanamine
C)N-Cyclohexylethanamine
D)N-Ethylcyclopentanamine
E)N-Ethylaniline

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of these is an acceptable alternative name to "(1-methylbutyl)amine"?
A)2-Aminopentane
B)2-Pentanamine
C)Isopentylamine
D)sec-Pentylamine
E)Both 2-aminopentane and 2-pentanamine
A)2-Aminopentane
B)2-Pentanamine
C)Isopentylamine
D)sec-Pentylamine
E)Both 2-aminopentane and 2-pentanamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
15
What type of amine is indole?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
16
What type of amine is N-methylmorpholine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
17
What type of amine is N-methyl-2-methyl-3-hexanamine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following is a tertiary amine?
A)CH3CH2CH2CH2NH2
B)CH3CH2NHCH2CH(CH3)2
C)(CH3CH2)2NCH2CH(CH3)2
D)(CH3CH2)4N+ OH-
E)(CH3CH2)3CNH2
A)CH3CH2CH2CH2NH2
B)CH3CH2NHCH2CH(CH3)2
C)(CH3CH2)2NCH2CH(CH3)2
D)(CH3CH2)4N+ OH-
E)(CH3CH2)3CNH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
19
The aromatic amine imidazole contains how many nitrogen atoms?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
20
What type of amine is pyrrolidine?
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.
A)Primary
B)Secondary
C)Tertiary
D)Quaternary
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of these alkyl halides cannot be used effectively in a Gabriel amine synthesis?
A)1-bromopentane
B)1-bromo-3-methylbutane
C)2-bromo-3-methylpentane
D)1-bromo-2,3-dimethylbutane
E)2-bromo-2,3-dimethylbutane
A)1-bromopentane
B)1-bromo-3-methylbutane
C)2-bromo-3-methylpentane
D)1-bromo-2,3-dimethylbutane
E)2-bromo-2,3-dimethylbutane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
22
Compound W has the molecular formula C11H17N.Treatment of W with benzenesulfonyl chloride in base gives no reaction.Acidification of the resulting mixture gives a clear solution.The 1H NMR spectrum of W consists of: triplet, 1.0
Quartet, 2.5
Singlet, 3.6 (2H)
Multiplet, 7.3 (5H)
The most likely structure for W is:
A)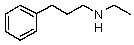
B)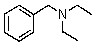
C)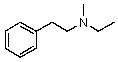
D)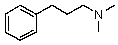
E)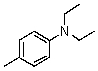
Quartet, 2.5
Singlet, 3.6 (2H)
Multiplet, 7.3 (5H)
The most likely structure for W is:
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which reagent will distinguish between 2-amino-2,3-dimethylpentane and 1-amino-3-methyl-2-pentene?
A)HONO,0-5 °C
B)C6H5SO2Cl/OH-,then H3O+
C)NaOH
D)HCl
E)Br2/CCl4
A)HONO,0-5 °C
B)C6H5SO2Cl/OH-,then H3O+
C)NaOH
D)HCl
E)Br2/CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 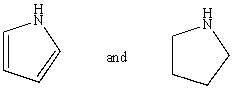
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl
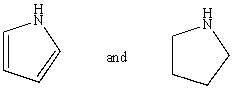
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
25
The reaction of which of these compounds with nitrous acid results in a stable N-nitroso compound?
A)C6H5NH2
B)C6H5N(CH3)2
C)CH3CH2CH2CH2CH2NH2
D)C6H5NHCH3
E)CH3CH2CONH2
A)C6H5NH2
B)C6H5N(CH3)2
C)CH3CH2CH2CH2CH2NH2
D)C6H5NHCH3
E)CH3CH2CONH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
26
This type of compound is the only one of these which can be converted by reduction into a 1 ,2 or 3 amine,according to its particular structure:
A)Nitrile
B)Oxime
C)Azide
D)Amide
E)Nitroalkane
A)Nitrile
B)Oxime
C)Azide
D)Amide
E)Nitroalkane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
27
The correct structure of (R)-N-methylbicyclo[2.2.1]heptan-2-amine is? ![<strong>The correct structure of (R)-N-methylbicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8522_9180_4ff0b624f292_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure of (R)-N-methylbicyclo[2.2.1]heptan-2-amine is? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3e2f_8522_9180_4ff0b624f292_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 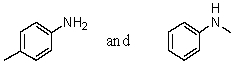
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl
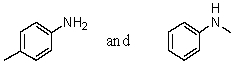
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 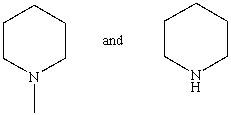
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 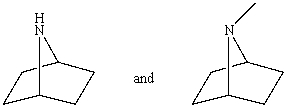
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
32
When the process ArNH2 ArY is carried out via an intermediate diazonium salt,this salt is isolated only in the case in which Y is which of these groups?
A)-F
B)-Cl
C)-Br
D)-I
E)-CN
A)-F
B)-Cl
C)-Br
D)-I
E)-CN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following compounds would be the strongest base? 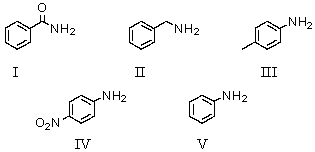
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the basis for the successful resolution of racemic C6H5CHOHCO2H through use of the chiral amine,C6H5CH(NH2)CH3?
A)One enantiomer is more soluble than the other.
B)The racemic mixture is converted into a single isomer in the basic solvent.
C)The diastereomeric salts formed have different solubilities.
D)The diastereomeric salts have different boiling points.
E)The diastereomeric salts have different melting points.
A)One enantiomer is more soluble than the other.
B)The racemic mixture is converted into a single isomer in the basic solvent.
C)The diastereomeric salts formed have different solubilities.
D)The diastereomeric salts have different boiling points.
E)The diastereomeric salts have different melting points.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of these compounds is expected to possess the lowest boiling point?
A)CH3CH2CH2CH2CH2NH2
B)CH3CH2CH2NHCH2CH3
C)(CH3CH2)2NCH3
D)(CH3CH2)2CHOH
E)(CH3)3CCH2NH3+ Cl-
A)CH3CH2CH2CH2CH2NH2
B)CH3CH2CH2NHCH2CH3
C)(CH3CH2)2NCH3
D)(CH3CH2)2CHOH
E)(CH3)3CCH2NH3+ Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 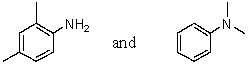
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl
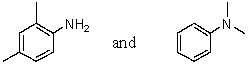
A)AgNO3 in H2O
B)Dilute NaHCO3
C)Dilute NaOH
D)C6H5SO2Cl/OH-,then H3O+
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which is not an intermediate in the Hofmann degradation reaction? 
A)RN=C=O
B)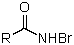
C)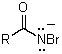
D)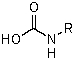
E)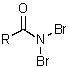
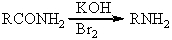
A)RN=C=O
B)

C)
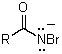
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which reagent could be used to separate a mixture of aniline and toluene?
A)KMnO4 in H2O
B)Dilute NaOH
C)Dilute NaHCO3
D)Ag(NH3)2OH
E)Dilute HCl
A)KMnO4 in H2O
B)Dilute NaOH
C)Dilute NaHCO3
D)Ag(NH3)2OH
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 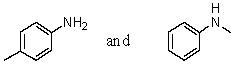
A)Tollens' test
B)Dilute NaHCO3
C)Hinsberg test
D)Iodoform test
E)None of these choices.
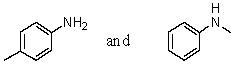
A)Tollens' test
B)Dilute NaHCO3
C)Hinsberg test
D)Iodoform test
E)None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which reagent would serve as the basis for a simple chemical test that would distinguish between the pair of compounds listed below? 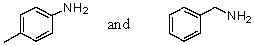
A)AgNO3 in H2O
B)HONO,0-5 °C then -naphthol
C)Dilute NaOH
D)C6H5SO2Cl and OH- in H2O
E)Dilute HCl
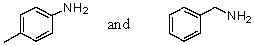
A)AgNO3 in H2O
B)HONO,0-5 °C then -naphthol
C)Dilute NaOH
D)C6H5SO2Cl and OH- in H2O
E)Dilute HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following aryl amines is the strongest base?
A)Aniline
B)p-Trimethylammonium aniline
C)p-Methoxyaniline
D)p-Trifluoromethylaniline
E)p-Chloroaniline
A)Aniline
B)p-Trimethylammonium aniline
C)p-Methoxyaniline
D)p-Trifluoromethylaniline
E)p-Chloroaniline

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following bases has a conjugate acid with the smallest pKa?
A)p-Methylaniline
B)p-Methoxyaniline
C)Hexylamine
D)p-Nitroaniline
E)Dipropylamine
A)p-Methylaniline
B)p-Methoxyaniline
C)Hexylamine
D)p-Nitroaniline
E)Dipropylamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
43
Arrange the following compounds in order of increasing basicity (least to most)in aqueous solution: 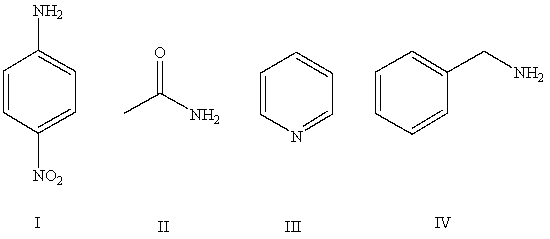
A)IV < II < I < III
B)III < II < IV < I
C)II < I < III < IV
D)II < III < I < IV
E)Cannot be determined from information given

A)IV < II < I < III
B)III < II < IV < I
C)II < I < III < IV
D)II < III < I < IV
E)Cannot be determined from information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of these would be predicted to have the largest pKa? 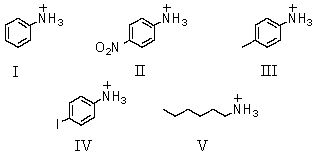
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of these is the weakest acid? 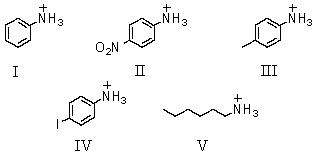
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
46
Arrange the following compounds in order of increasing basicity (least to most)in aqueous solution: 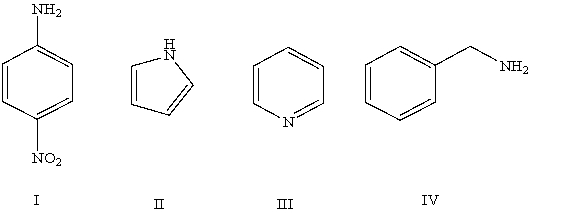
A)IV < II < I < III
B)III < II < IV < I
C)II < I < III < IV
D)II < III < I < IV
E)Cannot be determined from information given

A)IV < II < I < III
B)III < II < IV < I
C)II < I < III < IV
D)II < III < I < IV
E)Cannot be determined from information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
47
Arrange the following amines in order of decreasing basicity (most to least)in aqueous solution: 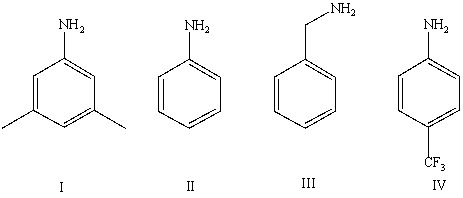
A)IV > II > I > III
B)III > II > IV > I
C)III > I > II > IV
D)II > III > I > IV
E)Cannot be determined from information given

A)IV > II > I > III
B)III > II > IV > I
C)III > I > II > IV
D)II > III > I > IV
E)Cannot be determined from information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of these would be predicted to have the smallest pKa? 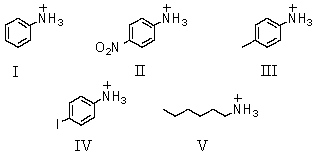
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following compounds would be the weakest base? 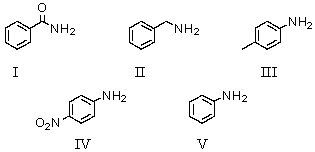
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following compounds would be the strongest base? 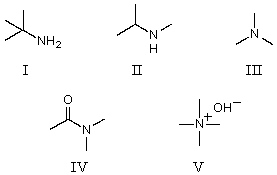
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following bases has a conjugate acid with the smallest pKa? 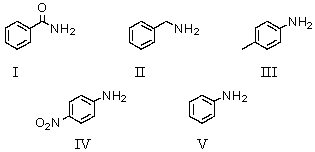
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following bases has a conjugate acid with the largest pKa? 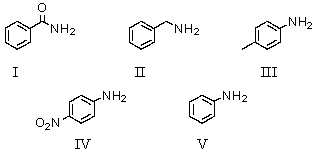
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following aryl amines is the weakest base?
A)Aniline
B)p-Trimethylammonium aniline
C)p-Methoxyaniline
D)p-Trifluoromethylaniline
E)p-Chloroaniline
A)Aniline
B)p-Trimethylammonium aniline
C)p-Methoxyaniline
D)p-Trifluoromethylaniline
E)p-Chloroaniline

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which would be the weakest base?
A)p-Methylaniline
B)p-Methoxyaniline
C)Hexylamine
D)p-Nitroaniline
E)Dipropylamine
A)p-Methylaniline
B)p-Methoxyaniline
C)Hexylamine
D)p-Nitroaniline
E)Dipropylamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
55
In aqueous solution,which of the following bases has the conjugate acid that possesses the smallest value for pKa?
A)C6H5NH2
B)NH3
C)(CH3CH2)3N
D)(CH3CH2)2NH
E)CH3CH2CH2NH2
A)C6H5NH2
B)NH3
C)(CH3CH2)3N
D)(CH3CH2)2NH
E)CH3CH2CH2NH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of these is the strongest acid? 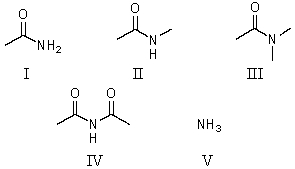
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following bases has a conjugate acid with the smallest pKa? 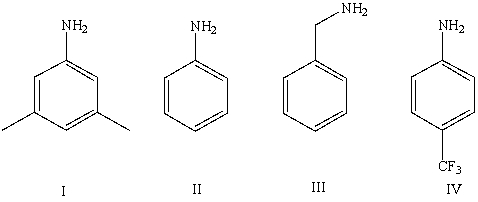
A)I
B)II
C)III
D)IV
E)Cannot be determined from information given

A)I
B)II
C)III
D)IV
E)Cannot be determined from information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of these is the strongest acid? 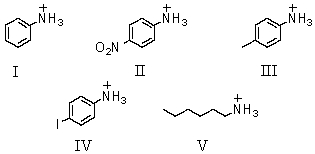
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
59
Arrange the following amines in order of increasing basicity (least to most)in aqueous solution: 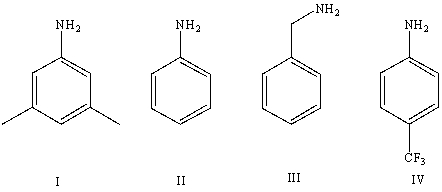
A)IV < II < I < III
B)III < II < IV < I
C)III < I < II < IV
D)II < III < I < IV
E)Cannot be determined from information given

A)IV < II < I < III
B)III < II < IV < I
C)III < I < II < IV
D)II < III < I < IV
E)Cannot be determined from information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of these compounds is soluble in dilute sodium hydroxide solution? 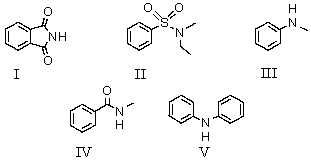
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
61
Identify the best method(s)to prepare 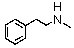 .
.
A)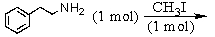
B)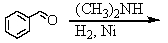
C)
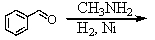
D)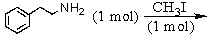 and
and
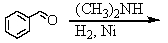
E)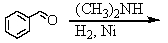 and
and
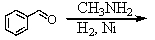
 .
.A)
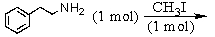
B)
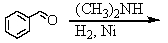
C)
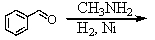
D)
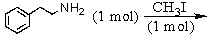 and
and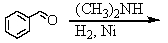
E)
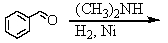 and
and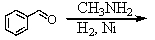

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following can be used to prepare allylamine (pure)? 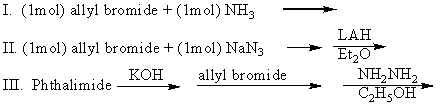
A)I
B)II
C)III
D)I and II
E)II and III
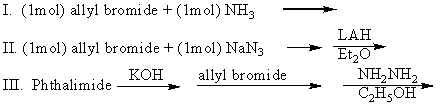
A)I
B)II
C)III
D)I and II
E)II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following can be used to prepare benzylamine (pure)? 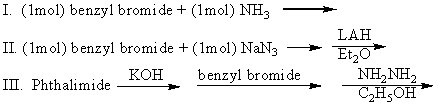
A)I
B)II
C)III
D)I and II
E)II and III
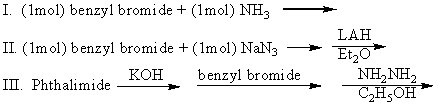
A)I
B)II
C)III
D)I and II
E)II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
64
Consider the synthesis below.What is reagent A? 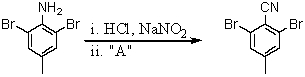
A)H3PO2
B)HCN
C)P4O10
D)CuCN
E)CuCl2
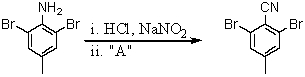
A)H3PO2
B)HCN
C)P4O10
D)CuCN
E)CuCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
65
Identify the best method(s)to prepare N-methylphenylmethanamine.
A)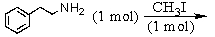
B)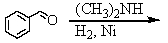
C)
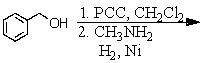
D)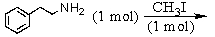 and
and
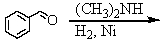
E)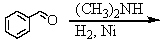 and
and
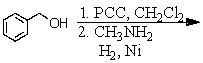
A)
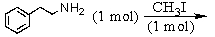
B)
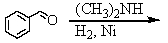
C)
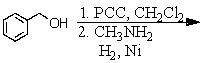
D)
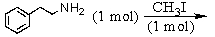 and
and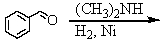
E)
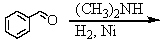 and
and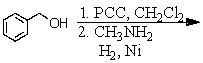

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
66
What reagent can effect the following transformation? 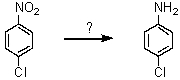
A)Sn/HCl; then OH-
B)NH2Cl
C)H3PO2
D)CuCN
E)HONO; then NH3

A)Sn/HCl; then OH-
B)NH2Cl
C)H3PO2
D)CuCN
E)HONO; then NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which is the best method to prepare 1-phenylethanamine?
A)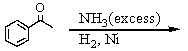
B)
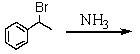
C)
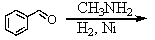
D)
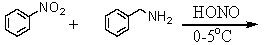
E)
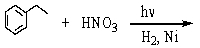
A)
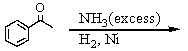
B)

C)
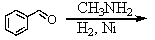
D)

E)
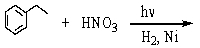

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
68
Identify the best method(s)to prepare N-methylphenylmethanamine.
A)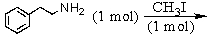
B)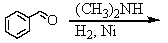
C)
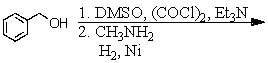
D)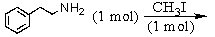 and
and
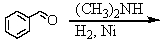
E)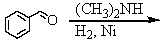 and
and
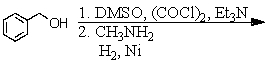
A)
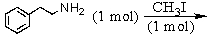
B)
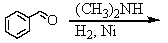
C)
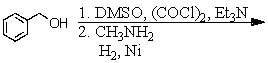
D)
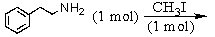 and
and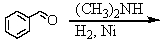
E)
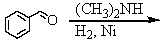 and
and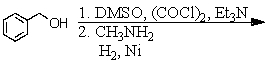

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
69
Consider the synthesis below.What is compound B? 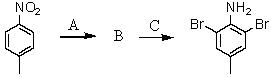
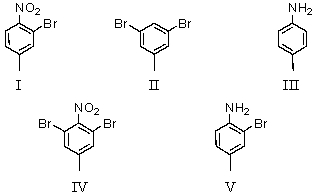
A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
70
What reagent can effect the following transformation? 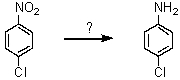
A)Fe/HCl; then OH-
B)NH2Cl
C)H3PO2
D)CuCN
E)HONO; then NH3

A)Fe/HCl; then OH-
B)NH2Cl
C)H3PO2
D)CuCN
E)HONO; then NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which is the best method to prepare 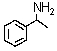 ?
?
A)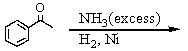
B)
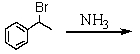
C)
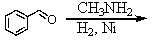
D)
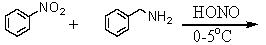
E)
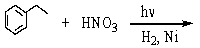
 ?
?A)
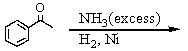
B)

C)
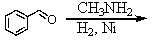
D)

E)
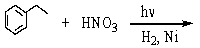

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
72
Consider the synthesis below.What is reagent "Y"? 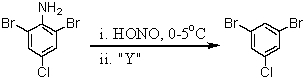
A)Br2/FeBr3
B)CuBr
C)CuBr2
D)H3PO2/H2O
E)H3PO4
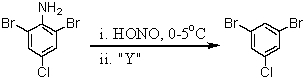
A)Br2/FeBr3
B)CuBr
C)CuBr2
D)H3PO2/H2O
E)H3PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
73
Identify the best method(s)to prepare N-methylphenylmethanamine.
A)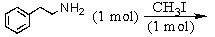
B)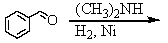
C)
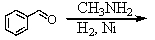
D)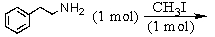 and
and
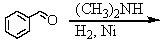
E)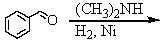 and
and
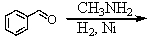
A)
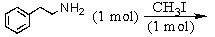
B)
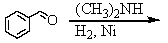
C)
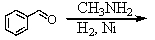
D)
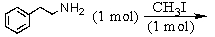 and
and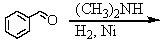
E)
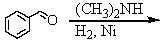 and
and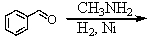

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which is the best method to prepare 1-phenylethanamine?
A)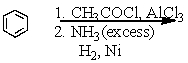
B)
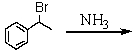
C)
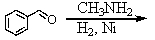
D)
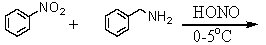
E)
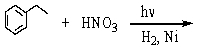
A)
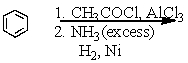
B)

C)
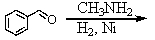
D)

E)
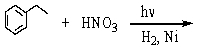

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of the following reactions would yield C6H5CH2NH2?
A)
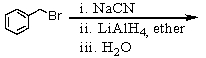
B)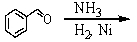
C)
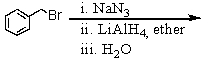
D)All of these choices.
E)Two of these choices.
A)
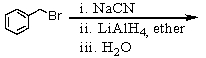
B)
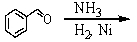
C)
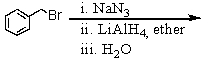
D)All of these choices.
E)Two of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
76
Consider the synthesis below.What is reagent A? 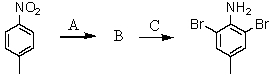
A)Br2,FeBr3
B)Fe,HCl; then OH-
C)NH2Cl,AlCl3
D)H3PO2
E)LiNH2

A)Br2,FeBr3
B)Fe,HCl; then OH-
C)NH2Cl,AlCl3
D)H3PO2
E)LiNH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
77
Consider the synthesis below.What is reagent "Z"? 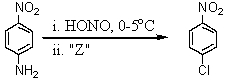
A)CuCl
B)CuCl2
C)NaCl
D)KCl
E)HCl/heat
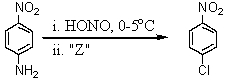
A)CuCl
B)CuCl2
C)NaCl
D)KCl
E)HCl/heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of these could be resolved into separate enantiomers?
A)4-Methyl-1-pentanamine
B)4-Methyl-2-pentanamine
C)N-Methyl-1-butanamine
D)N,N-Dimethyl-1-propanamine
E)N-Butyltrimethylammonium bromide
A)4-Methyl-1-pentanamine
B)4-Methyl-2-pentanamine
C)N-Methyl-1-butanamine
D)N,N-Dimethyl-1-propanamine
E)N-Butyltrimethylammonium bromide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
79
Consider the synthesis below.What is reagent C? 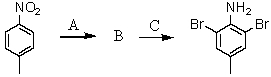
A)excess Br2,H2O
B)Fe,HCl; then OH-
C)NH2Cl,AlCl3
D)CuBr
E)HNO3,H2SO4,Fe

A)excess Br2,H2O
B)Fe,HCl; then OH-
C)NH2Cl,AlCl3
D)CuBr
E)HNO3,H2SO4,Fe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which of the following can be used to prepare 2-aminopentane (pure)? 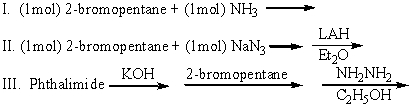
A)I
B)II
C)III
D)I and II
E)II and III
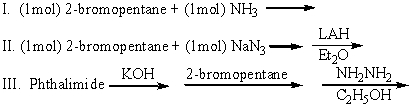
A)I
B)II
C)III
D)I and II
E)II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 207 في هذه المجموعة.
فتح الحزمة
k this deck








