Deck 9: Acids, bases, and Salts
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/86
العب
ملء الشاشة (f)
Deck 9: Acids, bases, and Salts
1
According to the Arrhenius theory,what is produced when an acid dissolves in water?
A)H+
B)OH-
C)H3O+
D)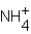
A)H+
B)OH-
C)H3O+
D)
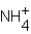
H+
2
What is the function of 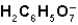 in the first ionization of citric acid?
in the first ionization of citric acid? 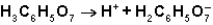
A)The ion serves as an Arrhenius acid in this reaction.
B)The ion serves as an Arrhenius base in this reaction.
C)The ion serves as the conjugate base of the acid,H3C6H5O7.
D)The ion serves as the conjugate acid of the base,H3C6H5O7.
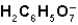 in the first ionization of citric acid?
in the first ionization of citric acid? 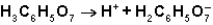
A)The ion serves as an Arrhenius acid in this reaction.
B)The ion serves as an Arrhenius base in this reaction.
C)The ion serves as the conjugate base of the acid,H3C6H5O7.
D)The ion serves as the conjugate acid of the base,H3C6H5O7.
The ion serves as the conjugate base of the acid,H3C6H5O7.
3
Identify the Brønsted acid(s)in the reaction. HIO3(aq)+ H2O (l)  H3O+ (aq)+ IO3- (aq)
H3O+ (aq)+ IO3- (aq)
A)HIO3
B)HIO3 and H3O+
C)H2O
D)H2O and HIO3
 H3O+ (aq)+ IO3- (aq)
H3O+ (aq)+ IO3- (aq)A)HIO3
B)HIO3 and H3O+
C)H2O
D)H2O and HIO3
HIO3 and H3O+
4
The molar concentration of H+ ions in a solution is 5.8 *10-9.The pH is _____ .
A)9.00
B)5.80
C)between 8.00 and 9.00
D)between 9.00 and 10.00
A)9.00
B)5.80
C)between 8.00 and 9.00
D)between 9.00 and 10.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following substances could behave as an Arrhenius base?
A)Na2CO3
B)Na3PO4
C)K2S
D)KOH
A)Na2CO3
B)Na3PO4
C)K2S
D)KOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
6
A water solution is found to have a molar OH- concentration of 3.2 *10-5.The solution would be classified as _____ .
A)acidic
B)basic
C)neutral
D)can't be classified
A)acidic
B)basic
C)neutral
D)can't be classified

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements is true for neutral solutions in which water is the solvent?
A)Neutral solutions contain no H3O+ ions.
B)Neutral solutions contain no OH - ions.
C)Neutral solutions contain H3O+ and OH - ions in equal concentrations.
D)More than one response is correct.
A)Neutral solutions contain no H3O+ ions.
B)Neutral solutions contain no OH - ions.
C)Neutral solutions contain H3O+ and OH - ions in equal concentrations.
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
8
The cation (positive ion)in a salt comes from _____ .
A)an acid
B)a base
C)either an acid or a base
D)water
A)an acid
B)a base
C)either an acid or a base
D)water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
9
In the Brønsted theory,both acids and bases are defined in terms of how substances lose or gain
A)OH-.
B)H3O+.
C)H+.
D)Cl -
A)OH-.
B)H3O+.
C)H+.
D)Cl -

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following is present in pure water?
A)H3O+
B)OH -
C)H2O
D)all of them
A)H3O+
B)OH -
C)H2O
D)all of them

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
11
What are the missing products in the following reaction when it is written as a full equation? 2HBr + SrCO3 ____ + ____ + H2O
A)SrBr2 + CO2
B)SrBr2 +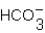
C)Sr2 + H2CO3
D)HBr + CO2
A)SrBr2 + CO2
B)SrBr2 +
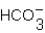
C)Sr2 + H2CO3
D)HBr + CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
12
What salt would be produced by the reaction of H2SO4 with LiHCO3?
A)Li2S
B)LiSO4
C)Li2SO4
D)Li2CO3
A)Li2S
B)LiSO4
C)Li2SO4
D)Li2CO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following is a property of acid solutions?
A)sour taste
B)slippery feel
C)change red litmus to blue
D)More than one response is correct.
A)sour taste
B)slippery feel
C)change red litmus to blue
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
14
Many cleaning agents contain bases because bases react with _____ .
A)fats or oils
B)glass
C)salts
D)More than one response is correct.
A)fats or oils
B)glass
C)salts
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
15
The molar concentration of OH in a water solution is 1.0 * 10-9.The pH of the solution is _____ .
A)9.00
B)5.00
C)> 9.00
D)< 5.00
A)9.00
B)5.00
C)> 9.00
D)< 5.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
16
What volume of 6.0 M HNO3 would be needed to make 500 mL of 0.50 M solution?
A)6 mL
B)417 mL
C)42 mL
D)6 liters
A)6 mL
B)417 mL
C)42 mL
D)6 liters

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
17
Identify all Brønsted base(s)in the reaction. N3- (aq)+ H2O (l)  HN3 (aq)+ OH- (aq)
HN3 (aq)+ OH- (aq)
A)H2O
B)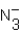
C)OH-
D)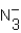 and OH-
and OH-
 HN3 (aq)+ OH- (aq)
HN3 (aq)+ OH- (aq)A)H2O
B)
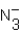
C)OH-
D)
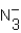 and OH-
and OH-
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
18
A solution for which [H+] = 1.0 *10-3 will have a pH of _____ .
A)"5.00"
B)"3.00"
C)"-05.00"
D)"-9.00"
A)"5.00"
B)"3.00"
C)"-05.00"
D)"-9.00"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
19
Some salts isolated by evaporation retain water that is called
A)water of hydration.
B)hydroxide.
C)hydronium.
D)crystallized water.
A)water of hydration.
B)hydroxide.
C)hydronium.
D)crystallized water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
20
In the following reaction,all substances are soluble,and all dissociate except H2O.Identify the spectator ions.HCl + KOH KCl + H2O
A)H+ and OH+
B)K+ and Cl-
C)H+ and K+
D)Cl- and OH-
A)H+ and OH+
B)K+ and Cl-
C)H+ and K+
D)Cl- and OH-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
21
When an acid is analyzed by adding a measured quantity of base,the point at which all the acid has reacted is correctly called
A)the equivalence point.
B)the neutral point.
C)the endpoint.
D)the analysis point.
A)the equivalence point.
B)the neutral point.
C)the endpoint.
D)the analysis point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
22
The procedure commonly used to determine the amount of base in a solution by adding a measured quantity of acid is called _____ .
A)dissociation
B)titration
C)ionization
D)normalization
A)dissociation
B)titration
C)ionization
D)normalization

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which salt shifts the pH when dissolved in water?
A)KCl
B)Al(NO3)3
C)NaCl
D)NaNO3
A)KCl
B)Al(NO3)3
C)NaCl
D)NaNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
24
What would be the pH of a solution of NH4Cl in pure water?
A)higher than 7
B)lower than 7
C)equal to 7
D)can't be predicted
A)higher than 7
B)lower than 7
C)equal to 7
D)can't be predicted

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
25
A 25.00 mL sample of H2SO4 acid solution requires 17.35 mL of 0.118 N base for titration.What is the normality of the acid solution?
A)0.0819 N
B)0.164 N
C)0.0409 N
D)0.236 N
A)0.0819 N
B)0.164 N
C)0.0409 N
D)0.236 N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
26
A solution has a pH of 11.60.The value of [H+] is _____ .
A)2.5 * 10-4
B)4.0 * 10-3
C)4.0 * 10-11
D)2.5 * 10-12
A)2.5 * 10-4
B)4.0 * 10-3
C)4.0 * 10-11
D)2.5 * 10-12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
27
The classification of an acid or base as weak or strong is based upon
A)the solubility of the acid or base.
B)the extent of dissociation of the acid or base.
C)the concentration of acid or base in solution.
D)More than one response is correct.
A)the solubility of the acid or base.
B)the extent of dissociation of the acid or base.
C)the concentration of acid or base in solution.
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
28
Identify the substance with the lowest pH.
A)orange juice
B)household ammonia
C)baking soda,NaHCO3
D)milk
A)orange juice
B)household ammonia
C)baking soda,NaHCO3
D)milk

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
29
Antacids contain a substance that neutralizes hydrochloric acid.What is a candidate for use as an antacid?
A)Al(NO3)3
B)MgCl2
C)Al2(SO4)3
D)MgCO3
A)Al(NO3)3
B)MgCl2
C)Al2(SO4)3
D)MgCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
30
A solution has a pH of 8.72.What is the value of [H+]?
A)5.2 * 10-6
B)1.9 *10-9
C)5.2 * 10-8
D)1.9 *10sup>-5
A)5.2 * 10-6
B)1.9 *10-9
C)5.2 * 10-8
D)1.9 *10sup>-5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
31
A higher pH corresponds to _____ .
A)a higher [H+]
B)a lower [H+]
C)no change in [OH+]
D)a lower [OH - ]
A)a higher [H+]
B)a lower [H+]
C)no change in [OH+]
D)a lower [OH - ]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
32
A 25.00 mL sample of hydrochloric acid solution,HCl,is titrated with 0.0512 M NaOH solution.The volume of NaOH solution required is 21.68 mL.What is the molarity of the HCl solution?
A)0.0444
B)0.0590
C)0.0295
D)0.0148
A)0.0444
B)0.0590
C)0.0295
D)0.0148

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
33
If a solution of acetic acid (a weak acid)is titrated with NaOH solution,what will be the pH at the equivalence point?
A)higher than 7
B)lower than 7
C)equal to 7
D)can't predict
A)higher than 7
B)lower than 7
C)equal to 7
D)can't predict

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following is a weak acid?
A)HNO3
B)HCl
C)H2CO3
D)H2SO4
A)HNO3
B)HCl
C)H2CO3
D)H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many equivalents are contained in 0.25 moles of H2SO4?
A)0.25
B)0.13
C)0.50
D)can vary
A)0.25
B)0.13
C)0.50
D)can vary

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following mixtures would represent a buffer?
A)sodium chloride/hydrochloric acid
B)sodium sulfate/sulfuric acid
C)sodium formate/formic acid
D)none of these
A)sodium chloride/hydrochloric acid
B)sodium sulfate/sulfuric acid
C)sodium formate/formic acid
D)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
37
A solution for which [OH-] = 3.0 * 10-10 is classified as _____ .
A)acidic
B)basic
C)neutral
D)More than one response is correct.
A)acidic
B)basic
C)neutral
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
38
Phosphoric acid,H3PO4,undergoes three dissociation reactions.Which of the three acids is the weakest?
A)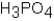
B)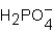
C)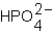
D)More than one response is correct.
A)
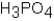
B)
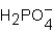
C)
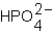
D)More than one response is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
39
In a buffer solution made up of equal concentrations of acetic acid and sodium acetate,NaC2H3O2,which component is used to buffer against added OH - ?
A)C2H3O2
B)Na+
C)OH -
D)HC2H3O2
A)C2H3O2
B)Na+
C)OH -
D)HC2H3O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following salts would produce a basic solution (pH higher than 7)upon being dissolved in pure,distilled water?
A)NaCl
B)Na2CO3
C)Mg(NO3)2
D)NH4Cl
A)NaCl
B)Na2CO3
C)Mg(NO3)2
D)NH4Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
41
The expression for the Ka of the weak acid HF would be which of the following?
A)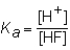
B)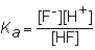
C)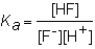
D)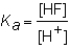
A)
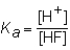
B)
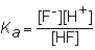
C)
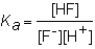
D)
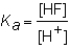

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
42
The buffer capacity is the amount of ____ that can be absorbed without causing significant changes in pH.
A)acid
B)base
C)neither acid or base
D)either acid or base
A)acid
B)base
C)neither acid or base
D)either acid or base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
43
A reaction in which an acid and a base react completely,leaving only a salt and water,is referred to as a(n)
A)hydrolysis reaction.
B)neutralization reaction.
C)oxidation reaction.
D)None of the choices.
A)hydrolysis reaction.
B)neutralization reaction.
C)oxidation reaction.
D)None of the choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
44
To determine the number of equivalents of Al(NO3)3 in a 0.750 M solution,the conversion factor would be which of the following?
A)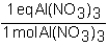
B)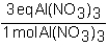
C)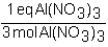
D)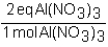
A)
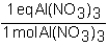
B)
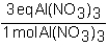
C)
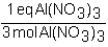
D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
45
HCl will react with
A)BaO
B)CaCO3
C)Mg
D)All three are correct.
A)BaO
B)CaCO3
C)Mg
D)All three are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
46
Identify two Brønsted base(s)in the reaction. HIO3 (aq)+ H2O (I)  H3O+ (aq)+ 103- (aq)
H3O+ (aq)+ 103- (aq)
A)HIO3,H2O
B)HIO3, H3O+
C)HIO3,IO3-
D)H2O,IO3-
 H3O+ (aq)+ 103- (aq)
H3O+ (aq)+ 103- (aq)A)HIO3,H2O
B)HIO3, H3O+
C)HIO3,IO3-
D)H2O,IO3-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the pKa of an acid with a Ka of 1.82* 10-5?
A)"4.74"
B)"-4.74"
C)"9.26"
D)"-9.26"
A)"4.74"
B)"-4.74"
C)"9.26"
D)"-9.26"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
48
When your liver detoxifies ethyl alcohol,the concentration of the hydrogen ion increases.This will result in
A)respiratory acidosis.
B)metabolic acidosis.
C)respiratory alkalosis.
D)metabolic alkalosis.
A)respiratory acidosis.
B)metabolic acidosis.
C)respiratory alkalosis.
D)metabolic alkalosis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
49
The dissociation reaction for the weak acid,H2PO3- would be which of the following?
A)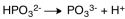
B)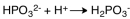
C)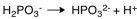
D)
A)
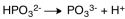
B)
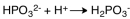
C)
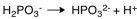
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
50
A 10-8 M solution of HCl is prepared.It would be expected to be _____.
A)basic
B)acidic
C)neutral
D)insufficient information
A)basic
B)acidic
C)neutral
D)insufficient information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
51
How many meq are contained in 25.00 mL of a 0.225 M solution of CaCl2?
A)5.62 * 10-3 meq
B)2.81 meq
C)1.12 * 10-2 meq
D)1.12 * 101 meq
A)5.62 * 10-3 meq
B)2.81 meq
C)1.12 * 10-2 meq
D)1.12 * 101 meq

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following would you expect to be a weak electrolyte?
A)acetic acid
B)sodium acetate
C)water
D)More than one answer is correct.
A)acetic acid
B)sodium acetate
C)water
D)More than one answer is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
53
A patient comes to you suffering from a battery acid burn (sulfuric acid).What is the best thing to use to neutralize the acid,while you continue to run cool water over the affected area?
A)a 1 M sodium hydroxide solution (NaOH)
B)a 1 M sodium bicarbonate solution (NaHCO3)
C)a 1 M acetic acid solution (CH3COOH)
D)a 1 M carbonic acid solution (H2CO3)
A)a 1 M sodium hydroxide solution (NaOH)
B)a 1 M sodium bicarbonate solution (NaHCO3)
C)a 1 M acetic acid solution (CH3COOH)
D)a 1 M carbonic acid solution (H2CO3)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of the following sets of reactants could be used to prepare CaI2?
A)CaO and HI
B)Ca(OH)2 and HI
C)Ca and HI
D)Any of these could be used.
A)CaO and HI
B)Ca(OH)2 and HI
C)Ca and HI
D)Any of these could be used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
55
The term,strong acid,refers to
A)the number of hydrogens attached to the acid molecule.
B)the speed at which it will dissolve metal.
C)if it will cause burns to the skin.
D)the ability for the acid to completely dissociate in solution.
A)the number of hydrogens attached to the acid molecule.
B)the speed at which it will dissolve metal.
C)if it will cause burns to the skin.
D)the ability for the acid to completely dissociate in solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
56
When a solution of HNO3 is added to a solution of NaHCO3,the net ion equation for reaction that occurs is which of the following?
A)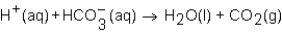
B)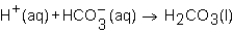
C)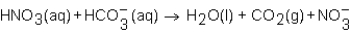
D)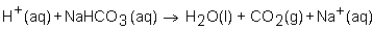
A)
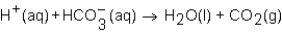
B)
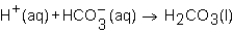
C)
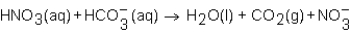
D)
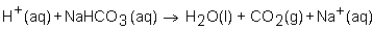

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
57
During a field trip to study the problem of acid rain,you test one lake that had observed a major fish kill.It was found to have a pH of 5.37.What is the [H+]?
A)2.3*10-9
B)3.7*10-5
C)4.3*10-6
D)1.7*105
A)2.3*10-9
B)3.7*10-5
C)4.3*10-6
D)1.7*105

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
58
Sodium phosphate can be used as a driveway degreaser.When added to water,the following equilibrium is attained.Sodium phosphate is acting as a(n)______. Na3PO4 (aq) + 3H2O (l)  H3PO4 (aq) + 3OH- (aq)
H3PO4 (aq) + 3OH- (aq)
A)Brønsted acid
B)Brønsted base
C)Arrhenius acid
D)Arrhenius base
 H3PO4 (aq) + 3OH- (aq)
H3PO4 (aq) + 3OH- (aq)A)Brønsted acid
B)Brønsted base
C)Arrhenius acid
D)Arrhenius base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
59
When a strong acid is added to a weak base and mixed,which of the following would not be produced?
A)conjugate acid
B)conjugate base
C)salt
D)water
A)conjugate acid
B)conjugate base
C)salt
D)water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
60
Consider the following list of pKa values for a series of acids.Which is the strongest acid?
A)5.74
B)3.21
C)10.6
D)Cannot be determined.
A)5.74
B)3.21
C)10.6
D)Cannot be determined.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
61
The salt of a weak base with a weak base could behave as a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
62
The reason pure water has a pH of 7 is that there is the same concentration of hydrogen and hydroxide ions on the self-ionization of water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
63
KOH is a strong base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
64
The pH of household ammonia is expected to be above 7 because household ammonia is NH4OH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
65
Seageroic acid has a pKa of 8.23,whereas slabaughic acid has a pKa of 18.65.From these facts,which of the following is true?
A)Slabaughic acid is less water soluble than seageroic acid.
B)Seageroic acid is a stronger acid than slabaughic acid.
C)Slabaughic acid dissociates to a greater extent in water.
D)Seageroic acid is a weaker acid than slabaughic acid.
A)Slabaughic acid is less water soluble than seageroic acid.
B)Seageroic acid is a stronger acid than slabaughic acid.
C)Slabaughic acid dissociates to a greater extent in water.
D)Seageroic acid is a weaker acid than slabaughic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
66
According to the Brønsted Theory,a conjugate pair is composed of a Brønsted acid and a Brønsted base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
67
Sodium nitrate in water will produce a basic solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
68
Solutions with a pH less than 7.00 are basic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
69
The pH of a solution of baking soda,NaHCO3,would be expected to be above 7.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
70
Nitric acid,HNO3,is a weak acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
71
The major difference between the Arrhenius Theory and the Brønsted Theory is that there is no hydrogen in the Brønsted Theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
72
The salt of a strong acid and a weak base will give an acidic solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
73
It has been proposed that atmospheric CO2 could be reduced by absorption in to the Earth's oceans.Which of the following reactions would be involved?
A)CO2 (g)+ H2O (l) H2CO3 (aq)
H2CO3 (aq)
B)H2CO3 (aq) H+ (aq)+ HCO3- (aq)
H+ (aq)+ HCO3- (aq)
C)HCO3- (aq) H+ (aq)+ CO32- (aq)
H+ (aq)+ CO32- (aq)
D)all of the above
A)CO2 (g)+ H2O (l)
 H2CO3 (aq)
H2CO3 (aq)B)H2CO3 (aq)
 H+ (aq)+ HCO3- (aq)
H+ (aq)+ HCO3- (aq)C)HCO3- (aq)
 H+ (aq)+ CO32- (aq)
H+ (aq)+ CO32- (aq)D)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
74
Ammonium chloride in water will produce an acidic solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
75
NaH2PO4 alone in a water solution could behave as a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
76
A Brønsted base is a proton donor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
77
The salt of a strong acid with a strong base could behave as a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
78
NaCl is an example of a good buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
79
Most acids are weak acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck
80
pH changes can be determined with a(n)___.
A)pH meter
B)chemical indicator
C)indicating paper
D)all of the above
A)pH meter
B)chemical indicator
C)indicating paper
D)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 86 في هذه المجموعة.
فتح الحزمة
k this deck








