Deck 12: Thermodynamics-Why Chemical Reactions Happen
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/152
العب
ملء الشاشة (f)
Deck 12: Thermodynamics-Why Chemical Reactions Happen
1
Which of the following statements regarding spontaneous processes is NOT true?
A)Spontaneous processes are exothermic.
B)Spontaneous processes proceed without outside intervention once initiated.
C)Spontaneous reactions are not always rapid.
D)The motional freedom of particles tends to increase during spontaneous change.
E)Particles tend to become more spread out during spontaneous change.
A)Spontaneous processes are exothermic.
B)Spontaneous processes proceed without outside intervention once initiated.
C)Spontaneous reactions are not always rapid.
D)The motional freedom of particles tends to increase during spontaneous change.
E)Particles tend to become more spread out during spontaneous change.
Spontaneous processes are exothermic.
2
Boltzmann derived the relationship S = k ln W,where W is the __________
A)number of microstates.
B)vibrational energy.
C)kinetic energy.
D)Wentworth factor.
E)potential energy.
A)number of microstates.
B)vibrational energy.
C)kinetic energy.
D)Wentworth factor.
E)potential energy.
number of microstates.
3
Of the three modes of molecular motion-vibration,rotation,and translation-which requires the greatest amount of energy to cause an excitation from the ground state to the first excited state?
A)vibration
B)rotation
C)translation
D)They all require the same amount of energy.
E)None,as quantized energy states do not apply to these motions.
A)vibration
B)rotation
C)translation
D)They all require the same amount of energy.
E)None,as quantized energy states do not apply to these motions.
vibration
4
Which of the following must be true for the microstates of a system?
I.The energy of each microstate equals the energy of the system.
II.The entropy of each microstate equals the entropy of the system.
III.The number of microstates equals the entropy of the system.
A)I only
B)II only
C)III only
D)I and III only
E)I,II,and III are all true
I.The energy of each microstate equals the energy of the system.
II.The entropy of each microstate equals the entropy of the system.
III.The number of microstates equals the entropy of the system.
A)I only
B)II only
C)III only
D)I and III only
E)I,II,and III are all true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
5
An oxygen molecule at room temperature can access the largest number of accessible microstates associated with which mode of molecular motion?
A)vibrational
B)kinetic
C)translational
D)elastic
E)Boltzmann
A)vibrational
B)kinetic
C)translational
D)elastic
E)Boltzmann

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following processes is NOT spontaneous?
A)Iron in moist air rusts.
B)Carbon dioxide and water react to form sugar and oxygen gas.
C)Liquid water in a freezer turns to ice.
D)A spark ignites a mixture of propane and air.
E)Baking soda (sodium hydrogen carbonate)reacts with lemon juice (dilute citric acid).
A)Iron in moist air rusts.
B)Carbon dioxide and water react to form sugar and oxygen gas.
C)Liquid water in a freezer turns to ice.
D)A spark ignites a mixture of propane and air.
E)Baking soda (sodium hydrogen carbonate)reacts with lemon juice (dilute citric acid).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following is the best definition of a microstate as it pertains to entropy on the molecular level?
A)the state (solid,liquid,or gas)of a small cluster of atoms or molecules
B)a property of a small cluster of atoms and molecules,such as temperature,that is independent of how the property was achieved
C)the set of vibrational,rotational,and translational energy levels in a system
D)a unique specification of the vibrational,rotational,and translational states of a single molecule in a system
E)a unique specification of the vibrational,rotational,and translational states of all of the particles in a system
A)the state (solid,liquid,or gas)of a small cluster of atoms or molecules
B)a property of a small cluster of atoms and molecules,such as temperature,that is independent of how the property was achieved
C)the set of vibrational,rotational,and translational energy levels in a system
D)a unique specification of the vibrational,rotational,and translational states of a single molecule in a system
E)a unique specification of the vibrational,rotational,and translational states of all of the particles in a system

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following statements regarding entropy is NOT true?
A)It is a measure of the distribution of energy in a system at a specific temperature.
B)It is a measure of the number of accessible microstates in a pure substance.
C)It is an index of the randomness of the particles in a system.
D)It is a property of the universe that increases during a spontaneous process.
E)It is a property of a system that may increase or decrease during a spontaneous process.
A)It is a measure of the distribution of energy in a system at a specific temperature.
B)It is a measure of the number of accessible microstates in a pure substance.
C)It is an index of the randomness of the particles in a system.
D)It is a property of the universe that increases during a spontaneous process.
E)It is a property of a system that may increase or decrease during a spontaneous process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
9
According to the second law of thermodynamics,the entropy change in an isolated system ( Ssys)during a spontaneous process must be __________
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than,less than,or equal to zero.
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than,less than,or equal to zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
10
The following figures represent distributions of two types of gas molecules between two containers connected by an open tube.In which figure is the entropy of the system maximized?
A)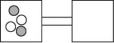
B)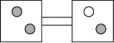
C)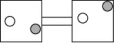
D)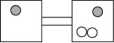
E)These are equal entropy systems.
A)

B)

C)

D)

E)These are equal entropy systems.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
11
When the volume of a gas is increased,the energy separation between microstates __________
A)increases.
B)decreases.
C)remains unchanged.
D)becomes infinite.
E)disappears.
A)increases.
B)decreases.
C)remains unchanged.
D)becomes infinite.
E)disappears.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
12
The following figures represent distributions of gas molecules between two containers connected by an open tube.In which figure is the entropy of the system maximized?
A)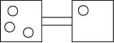
B)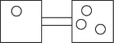
C)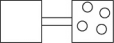
D)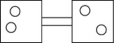
E)These are equal entropy systems.
A)

B)

C)

D)

E)These are equal entropy systems.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following is a driving force behind spontaneous reactions?
A)A solid is formed.
B)Heat is absorbed.
C)The reaction is rapid.
D)The motional freedom of particles increases.
E)The enthalpy of the particles increases.
A)A solid is formed.
B)Heat is absorbed.
C)The reaction is rapid.
D)The motional freedom of particles increases.
E)The enthalpy of the particles increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following states of motion has the highest number of occupied excited states at room temperature-rotational,vibrational,or translational?
A)rotational
B)vibrational
C)translational
D)The number of occupied excited states is the same for the three modes of motion.
E)None of these,as excited states do not apply to these motions.
A)rotational
B)vibrational
C)translational
D)The number of occupied excited states is the same for the three modes of motion.
E)None of these,as excited states do not apply to these motions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
15
What modes of motion does a molecule of chlorine have?
A)translational,rotational,and vibrational
B)kinetic energy and potential energy
C)microstate and macrostate
D)Boltzmann and non-Boltzmann
E)all of the above
A)translational,rotational,and vibrational
B)kinetic energy and potential energy
C)microstate and macrostate
D)Boltzmann and non-Boltzmann
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
16
The following figures represent distributions of gas molecules between two containers connected by an open tube.Which one represents the configuration with the largest number of equivalent microstates (most ways of achieving it)?
A)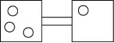
B)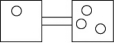
C)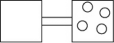
D)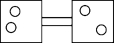
E)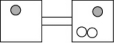
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
17
The following figures represent distributions of two types of gas molecules between two containers connected by an open tube.Which one represents the configuration with the largest number of equivalent microstates (most ways of achieving it)?
A)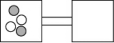
B)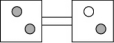
C)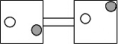
D)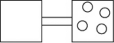
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
18
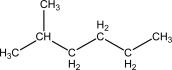
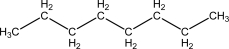
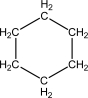
Given one mole of each of the following,which of the following has the highest number of accessible microstates?
A)Ice,H2O(s),at 273 K
B)Liquid water,H2O( ),at 373 K
),at 373 K
C)Steam,H2O(g),at 373 K
D)Propane,CH3CH2CH3(g),at 373 K
E)Butane,CH3CH2CH2CH3(g),at 373 K
A)Ice,H2O(s),at 273 K
B)Liquid water,H2O(
 ),at 373 K
),at 373 KC)Steam,H2O(g),at 373 K
D)Propane,CH3CH2CH3(g),at 373 K
E)Butane,CH3CH2CH2CH3(g),at 373 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
19
The entropy change in a system ( Ssys)during a spontaneous process must be __________
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than,less than,or equal to zero.
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than,less than,or equal to zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following statements is a valid definition of entropy?
A)It is a measure of the average kinetic energies of the particles in a system.
B)It is a measure of the enthalpy changes during a spontaneous change.
C)It is a measure of how the temperature changes during a spontaneous change.
D)It is a measure of how randomly arranged the particles in a system are.
E)It is a measure of how dispersed the energy of a system is.
A)It is a measure of the average kinetic energies of the particles in a system.
B)It is a measure of the enthalpy changes during a spontaneous change.
C)It is a measure of how the temperature changes during a spontaneous change.
D)It is a measure of how randomly arranged the particles in a system are.
E)It is a measure of how dispersed the energy of a system is.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
21
At 0 K,the entropy of a perfect crystal is __________
A)> 0
B)= 0
C)< 0
D)> 0,= 0,or < 0,depending on the chemical structure of the crystal.
E)> 0 or = 0,depending on the chemical structure of the crystal.
A)> 0
B)= 0
C)< 0
D)> 0,= 0,or < 0,depending on the chemical structure of the crystal.
E)> 0 or = 0,depending on the chemical structure of the crystal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following probably has the highest entropy at 298 K?
A)
B)
C)
D)
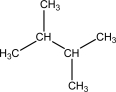
E)
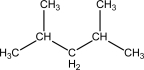
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following are listed in order of increasing standard molar entropy?
A)Au(s)< Au(g)< H2O( )< CH3OH(
)< CH3OH(  )< H2O(g)
)< H2O(g)
B)Au(s)< H2O( )< CH3OH(
)< CH3OH(  )< Au(g)< H2O(g)
)< Au(g)< H2O(g)
C)Au(s)< H2O( )< CH3OH(
)< CH3OH(  )< H2O(g)< Au(g)
)< H2O(g)< Au(g)
D)H2O( )< CH3OH(
)< CH3OH(  )< Au(s)< Au(g)< H2O(g)
)< Au(s)< Au(g)< H2O(g)
E)H2O( )< Au(s)< CH3OH(
)< Au(s)< CH3OH(  )< H2O(g)< Au(g)
)< H2O(g)< Au(g)
A)Au(s)< Au(g)< H2O(
 )< CH3OH(
)< CH3OH(  )< H2O(g)
)< H2O(g)B)Au(s)< H2O(
 )< CH3OH(
)< CH3OH(  )< Au(g)< H2O(g)
)< Au(g)< H2O(g)C)Au(s)< H2O(
 )< CH3OH(
)< CH3OH(  )< H2O(g)< Au(g)
)< H2O(g)< Au(g)D)H2O(
 )< CH3OH(
)< CH3OH(  )< Au(s)< Au(g)< H2O(g)
)< Au(s)< Au(g)< H2O(g)E)H2O(
 )< Au(s)< CH3OH(
)< Au(s)< CH3OH(  )< H2O(g)< Au(g)
)< H2O(g)< Au(g)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
24
Indicate which of the following has the highest entropy at 298 K.
A)0.5 g of HCN
B)1 mol of HCN
C)2 kg of HCN
D)2 mol of HCN
E)All of the above have the same entropy at 298 K.
A)0.5 g of HCN
B)1 mol of HCN
C)2 kg of HCN
D)2 mol of HCN
E)All of the above have the same entropy at 298 K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following statements regarding absolute and standard entropies is NOT true?
A)The standard entropy of a solution is the value of S when the solution is at 298 K and 1 M concentration.
B)The standard entropy of a gas is the value of S when the gas is at 298 K and 1 bar pressure.
C)The standard entropy of a pure solid or liquid is the value of S for its most stable allotrope at 298 K and 1 bar.
D)Elements in their standard states at 298 K and 1 bar have nonzero entropy values.
E)The entropy of a compound increases linearly with temperature.
A)The standard entropy of a solution is the value of S when the solution is at 298 K and 1 M concentration.
B)The standard entropy of a gas is the value of S when the gas is at 298 K and 1 bar pressure.
C)The standard entropy of a pure solid or liquid is the value of S for its most stable allotrope at 298 K and 1 bar.
D)Elements in their standard states at 298 K and 1 bar have nonzero entropy values.
E)The entropy of a compound increases linearly with temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following is in the correct order of standard state entropy?
I.Diamond < graphite
II.Liquid water < solid water
III.NH3 < H2
A)I only
B)II only
C)III only
D)I and II only
E)I and III only
I.Diamond < graphite
II.Liquid water < solid water
III.NH3 < H2
A)I only
B)II only
C)III only
D)I and II only
E)I and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
27
The molar entropies of carbon monoxide and carbon dioxide at 25 C are 197.7 and 213.8 J/mol . K,respectively.Which has more accessible microstates at 25 C? Approximately how many times more?
A)CO,roughly 16 times more
B)CO2,roughly 1.1 times more
C)CO2,roughly 1.3 1011 times more
D)CO2,roughly 6.9 times more
E)CO2,roughly 1.9 times more
A)CO,roughly 16 times more
B)CO2,roughly 1.1 times more
C)CO2,roughly 1.3 1011 times more
D)CO2,roughly 6.9 times more
E)CO2,roughly 1.9 times more

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
28
Indicate which of the following has the lowest standard molar entropy (S ).
A)CH4(g)
B)CH3CH2OH( )
)
C)H2O(s)
D)Na(s)
E)He(g)
A)CH4(g)
B)CH3CH2OH(
 )
)C)H2O(s)
D)Na(s)
E)He(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
29
The standard molar entropy of lead(II)bromide (PbBr2)is 161 J/mol . K.What is the entropy of 2.45 g of PbBr2?
A)+1.07 J/K
B)-1.07 J/K
C)+161 J/K
D)-161 J/K
E)0 J/K
A)+1.07 J/K
B)-1.07 J/K
C)+161 J/K
D)-161 J/K
E)0 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following is in the correct order of standard state entropy?
I.CO2(g)< CO(g)
II.Br2( )< Br2(g)
)< Br2(g)
III.S8(s)< S2(g)
A)I only
B)II only
C)III only
D)I and II only
E)II and III only
I.CO2(g)< CO(g)
II.Br2(
 )< Br2(g)
)< Br2(g)III.S8(s)< S2(g)
A)I only
B)II only
C)III only
D)I and II only
E)II and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
31
When carbon monoxide forms a crystal,each molecule can be pictured as having two possible orientations,CO or OC,because the molecular dipole moment is quite small.Assuming two possible microstates for each CO molecule,calculate the value of S when one mole of CO forms a crystalline solid at 0 K.
A)0 J/mol . K
B)2.00 J/mol . Kl
C)5.76 J/mol . K
D)9.57 10-23 J/mol . K
E)The value of S is too small to calculate.
A)0 J/mol . K
B)2.00 J/mol . Kl
C)5.76 J/mol . K
D)9.57 10-23 J/mol . K
E)The value of S is too small to calculate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
32
Perfect crystals of carbon monoxide (CO)are difficult to prepare because the very small dipole moment allows a few molecules to align in a pattern like CO OC CO instead of CO CO CO.If such crystals were cooled to 0 K,what would be the value of their absolute entropy?
A)> 0
B)= 0
C)< 0
D)> 0,= 0,or < 0,depending on how carefully it was cooled
E)> 0 or = 0,depending on how carefully it was cooled
A)> 0
B)= 0
C)< 0
D)> 0,= 0,or < 0,depending on how carefully it was cooled
E)> 0 or = 0,depending on how carefully it was cooled

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
33
At 25 C,a benzene molecule (C6H6)has approximately 1.143 1014 accessible microstates.Estimate the molar entropy of C6H6 at 25.0 C to two significant figures.
A)4.5 10-22 J/ mol . K
B)3.9 J/ mol . K
C)14 J/ mol . K
D)32 J/ mol . K
E)270 J/ mol . K
A)4.5 10-22 J/ mol . K
B)3.9 J/ mol . K
C)14 J/ mol . K
D)32 J/ mol . K
E)270 J/ mol . K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
34
Consider a closed container containing a 1.00 M solution of aqueous HCl at 298 K.Above the solution,air containing both water vapor and HCl gas at their equilibrium vapor pressures exerts a total pressure of 1.00 bar.Which of the following is/are in their thermodynamic standard state?
I.The liquid water
II.The HCl solution
III.The water vapor
A)I only
B)II only
C)III only
D)I and II only
E)I,II,and III are all in their standard state.
I.The liquid water
II.The HCl solution
III.The water vapor
A)I only
B)II only
C)III only
D)I and II only
E)I,II,and III are all in their standard state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many microstates are accessible to one mole of atoms in a perfect crystalline lattice at absolute zero?
A)0
B)1
C)1.381 10-23
D)52.64
E)6.022 1023
A)0
B)1
C)1.381 10-23
D)52.64
E)6.022 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
36
The molar entropy of ammonia,NH3(g),at 25 C is 192.5 J/mol . K.Estimate the number of microstates accessible to a NH3 molecule at 25 C to two significant figures.
A)1.1 1010
B)1.4 1025
C)6.8 1033
D)4.0 1083
E)23
A)1.1 1010
B)1.4 1025
C)6.8 1033
D)4.0 1083
E)23

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following will have the greatest standard molar entropy (S )?
A)NH3(g)
B)He(g)
C)C(s,graphite)
D)H2O( )
)
E)CaCO3(s)
A)NH3(g)
B)He(g)
C)C(s,graphite)
D)H2O(
 )
)E)CaCO3(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following graphs best depicts the entropy of a pure substance as the temperature is raised from its solid form through its liquid and gaseous forms? Assume the substance obeys the third law of thermodynamics.
A)
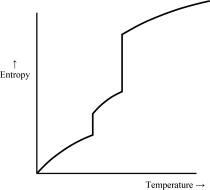
B)
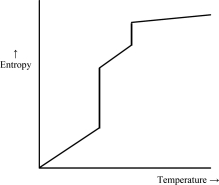
C)
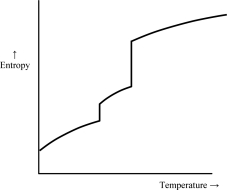
D)

E)
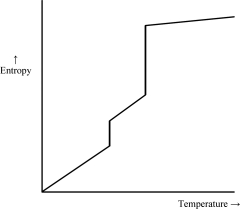
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
39
The absolute entropy of a NaCl crystal is __________
A)an intensive property and a state function.
B)an intensive property and a path function.
C)an extensive property and a state function.
D)an extensive property and a path function.
E)not appropriately described in terms of an intensive property,an extensive property,a state function,or a path function.
A)an intensive property and a state function.
B)an intensive property and a path function.
C)an extensive property and a state function.
D)an extensive property and a path function.
E)not appropriately described in terms of an intensive property,an extensive property,a state function,or a path function.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following processes is predicted to decrease the entropy of a system?
A)The number of particles increases.
B)Temperature increases.
C)Volume increases.
D)Pressure increases.
E)All of these increase the entropy of the system.
A)The number of particles increases.
B)Temperature increases.
C)Volume increases.
D)Pressure increases.
E)All of these increase the entropy of the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following processes are reversible in the thermodynamic sense?
I.Iron in the open air rusts.
II.NaCl is dissolved in water and then recovered by the evaporation of the water.
III.The ice in a mixture of ice and water at 0 C and 1 atm melts.
A)I only
B)II only
C)III only
D)II and III only
E)I,II,and III are all reversible.
I.Iron in the open air rusts.
II.NaCl is dissolved in water and then recovered by the evaporation of the water.
III.The ice in a mixture of ice and water at 0 C and 1 atm melts.
A)I only
B)II only
C)III only
D)II and III only
E)I,II,and III are all reversible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
42
During a spontaneous chemical reaction,it is found that Ssys is less than 0.This means __________
A)" Ssurr is less than 0 and its magnitude is less than Ssys."
B)" Ssurr is less than 0 and its magnitude is greater than Ssys."
C)" Ssurr is greater than 0 and its magnitude is less than Ssys."
D)" Ssurr is greater than 0 and its magnitude is greater than Ssys."
E)"an error has been made,as Ssys is greater than 0 by necessity for a spontaneous process."
A)" Ssurr is less than 0 and its magnitude is less than Ssys."
B)" Ssurr is less than 0 and its magnitude is greater than Ssys."
C)" Ssurr is greater than 0 and its magnitude is less than Ssys."
D)" Ssurr is greater than 0 and its magnitude is greater than Ssys."
E)"an error has been made,as Ssys is greater than 0 by necessity for a spontaneous process."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
43
If a reaction is spontaneous under a given set of conditions,__________
A)" Ssurr is greater than 0 for the reverse reaction."
B)"the reverse reaction is nonspontaneous."
C)" Ssys for the reverse reaction is smaller than Ssys for the forward reaction."
D)"the reverse reaction is also spontaneous."
E)"Suniv is greater than 0 for the reverse reaction."
A)" Ssurr is greater than 0 for the reverse reaction."
B)"the reverse reaction is nonspontaneous."
C)" Ssys for the reverse reaction is smaller than Ssys for the forward reaction."
D)"the reverse reaction is also spontaneous."
E)"Suniv is greater than 0 for the reverse reaction."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
44
The enthalpy of vaporization of ammonia at its normal boiling point,-33.34 C,is approximately 23.35 kJ/mol.What is the entropy change in the surroundings when one mole of liquid ammonia vaporizes at -33.34 C in a large room maintained at a temperature of 25.0 C?
A)-2.40 102 J/K
B)-78.3 J/K
C)-19.1 J/K
D)+78.3 J/K
E)+2.40 102 J/K
A)-2.40 102 J/K
B)-78.3 J/K
C)-19.1 J/K
D)+78.3 J/K
E)+2.40 102 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following statements regarding reversible processes is NOT true?
A)Heat transfer between the system and surroundings usually occurs reversibly during ordinary chemical reactions.
B)The change in entropy of the universe is zero during a reversible process.
C)The entropy change in the surroundings is equal and opposite to that of the system during a reversible process.
D)Reversible change would require an infinite amount of time to complete.
E)Heat transfer between the system and surroundings during phase changes can be approximated as reversible.
A)Heat transfer between the system and surroundings usually occurs reversibly during ordinary chemical reactions.
B)The change in entropy of the universe is zero during a reversible process.
C)The entropy change in the surroundings is equal and opposite to that of the system during a reversible process.
D)Reversible change would require an infinite amount of time to complete.
E)Heat transfer between the system and surroundings during phase changes can be approximated as reversible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
46
Heat transfer from the system to the surroundings has a large effect on Ssurr __________
A)when the temperature of the surroundings is low.
B)when the temperature of the surroundings is high.
C)when the temperature of the system is low.
D)when the temperature of the system is high.
E)at any temperature,as the amount of heat transferred is independent of temperature.
A)when the temperature of the surroundings is low.
B)when the temperature of the surroundings is high.
C)when the temperature of the system is low.
D)when the temperature of the system is high.
E)at any temperature,as the amount of heat transferred is independent of temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
47
During which of the following processes does the entropy of the system decrease?
A)Salt crystals dissolve in water.
B)Air escapes from a hole in a balloon.
C)Iron and oxygen gas react to form rust.
D)Ice melts in your hand.
E)None of these decrease the entropy of the system.
A)Salt crystals dissolve in water.
B)Air escapes from a hole in a balloon.
C)Iron and oxygen gas react to form rust.
D)Ice melts in your hand.
E)None of these decrease the entropy of the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
48
The entropy change of the surroundings, Ssurr,is related to heat transfer q with respect to the system and temperature T by __________
A)-q/Tsys = Ssurr.
B)+q/Tsys = Ssurr.
C)-q/Tsurr = Ssurr.
D)q/Tsurr = Ssurr.
E)None of these,unless the system undergoes a reversible process.
A)-q/Tsys = Ssurr.
B)+q/Tsys = Ssurr.
C)-q/Tsurr = Ssurr.
D)q/Tsurr = Ssurr.
E)None of these,unless the system undergoes a reversible process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
49
In a spontaneous process,which of the following always increases?
A)the entropy of the system
B)the entropy of the surroundings
C)the entropy of the universe
D)the entropy of the system and the universe
E)the entropy of the system,surroundings,and universe
A)the entropy of the system
B)the entropy of the surroundings
C)the entropy of the universe
D)the entropy of the system and the universe
E)the entropy of the system,surroundings,and universe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
50
According to the second law of thermodynamics,the change in the entropy of the universe ( Suniv)during a spontaneous reaction is __________
A)zero.
B)negative.
C)positive.
D)less than the change in entropy of the system ( Ssys).
E)greater than the change in entropy of the system ( Ssys).
A)zero.
B)negative.
C)positive.
D)less than the change in entropy of the system ( Ssys).
E)greater than the change in entropy of the system ( Ssys).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
51
When solid pellets of sodium hydroxide (NaOH)dissolve in water,the temperature of the water can rise dramatically.Taking NaOH as the system,what can you deduce about the signs of the entropy change of the system ( Ssys)and surroundings ( Ssurr)from this?
A)" Ssys is less than 0 and Ssurr is less than 0."
B)" Ssys is less than 0 and Ssurr is greater than 0."
C)" Ssys is greater than 0 and Ssurr is less than 0."
D)" Ssys is greater than 0 and Ssurr is greater than 0."
E)"Nothing can be deduced from this limited information."
A)" Ssys is less than 0 and Ssurr is less than 0."
B)" Ssys is less than 0 and Ssurr is greater than 0."
C)" Ssys is greater than 0 and Ssurr is less than 0."
D)" Ssys is greater than 0 and Ssurr is greater than 0."
E)"Nothing can be deduced from this limited information."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
52
The enthalpy and entropy of fusion of ice at 0.00 C are 6.01 kJ/mol and 22.0 J/mol . K,respectively.What is the entropy change of the universe when one mole of ice melts in a large room maintained at 34.00 C? Assume the final temperature of the water is 0.00 C.
A)+19.6 J/K
B)-19.6 J/K
C)+2.4 J/K
D)-2.4 J/K
E)+41.5 J/K
A)+19.6 J/K
B)-19.6 J/K
C)+2.4 J/K
D)-2.4 J/K
E)+41.5 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
53
Ssys can be directly related to the heat q.Which of the following is NOT true regarding this relationship? (If all are true,select E.)
A)" Ssys can always be determined from the heat transferred during the actual process."
B)"For a reversible spontaneous endothermic process,both q and Ssys will be positive."
C)"The more heat that is transferred,the larger the magnitude of the entropy change."
D)"The higher the temperature at which heat is transferred,the lower the entropy change."
E)"All of the above are true."
A)" Ssys can always be determined from the heat transferred during the actual process."
B)"For a reversible spontaneous endothermic process,both q and Ssys will be positive."
C)"The more heat that is transferred,the larger the magnitude of the entropy change."
D)"The higher the temperature at which heat is transferred,the lower the entropy change."
E)"All of the above are true."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
54
The enthalpy of vaporization of water at 100.0 C is 40.68 kJ/mol.What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 C in a large room maintained at a temperature of 25.00 C?
A)-136.4 J/K
B)-109.0 J/K
C)-40.68 J/K
D)+109.0 J/K
E)+136.4 J/K
A)-136.4 J/K
B)-109.0 J/K
C)-40.68 J/K
D)+109.0 J/K
E)+136.4 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following must be true for a spontaneous exothermic process?
A)only Ssys is less than 0
B)only Ssys is greater than 0
C)both Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr
D)both Ssys is less than 0 and the magnitude of Ssys is greater than the magnitude of Ssurr
E)either Ssys is greater than 0 or Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr
A)only Ssys is less than 0
B)only Ssys is greater than 0
C)both Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr
D)both Ssys is less than 0 and the magnitude of Ssys is greater than the magnitude of Ssurr
E)either Ssys is greater than 0 or Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
56
The gas above the liquid in a sealed bottle of soda is primarily carbon dioxide.Carbon dioxide is also dissolved in the soda.When the distribution of carbon dioxide between the gas and liquid is at equilibrium,molecules of carbon dioxide in the gas phase can still dissolve in the liquid phase if they strike the surface and are captured.Similarly,molecules of carbon dioxide can escape from the liquid phase.What is the entropy change of the universe, Suniv,for the dissolution of carbon dioxide under these conditions?
A)" Suniv is less than 0 because the dissolved carbon dioxide has fewer accessible states."
B)" Suniv is greater than 0 because the dissolved carbon dioxide has fewer accessible states."
C)" Suniv equals 0 because this is an equilibrium situation."
D)" Suniv is less than 0 because the gas dissolves spontaneously."
E)" Suniv is greater than 0 because the gas dissolves spontaneously."
A)" Suniv is less than 0 because the dissolved carbon dioxide has fewer accessible states."
B)" Suniv is greater than 0 because the dissolved carbon dioxide has fewer accessible states."
C)" Suniv equals 0 because this is an equilibrium situation."
D)" Suniv is less than 0 because the gas dissolves spontaneously."
E)" Suniv is greater than 0 because the gas dissolves spontaneously."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
57
When solid ammonium nitrate (NH4NO3)dissolves in water,the temperature of the water can drop dramatically.Taking NH4NO3 as the system,predict the signs of the entropy change of the system ( Ssys)and surroundings ( Ssurr).
A)" Ssys is less than 0 and Ssurr is less than 0."
B)" Ssys is less than 0 and Ssurr is greater than 0."
C)" Ssys is greater than 0 and Ssurr is less than 0."
D)" Ssys is greater than 0 and Ssurr is greater than 0."
E)" Ssys equals 0 and Ssurr is less than 0."
A)" Ssys is less than 0 and Ssurr is less than 0."
B)" Ssys is less than 0 and Ssurr is greater than 0."
C)" Ssys is greater than 0 and Ssurr is less than 0."
D)" Ssys is greater than 0 and Ssurr is greater than 0."
E)" Ssys equals 0 and Ssurr is less than 0."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the entropy change in the surroundings when one mole of ice melts at 0.00 C in a large room maintained at 32.0 C? The heat of fusion of ice is 6.01 kJ/mol.
A)-188 J/K
B)-22.0 J/K
C)-19.7 J/K
D)+19.7 J/K
E)+188 J/K
A)-188 J/K
B)-22.0 J/K
C)-19.7 J/K
D)+19.7 J/K
E)+188 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
59
In a spontaneous process,the entropy of the universe __________
A)always increases.
B)always decreases.
C)does not change.
D)may decrease if the entropy of the system decreases sufficiently.
E)may decrease if the entropy of the system increases sufficiently.
A)always increases.
B)always decreases.
C)does not change.
D)may decrease if the entropy of the system decreases sufficiently.
E)may decrease if the entropy of the system increases sufficiently.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
60
During which of the following processes does the entropy of the system increase?
A)Water vapor condenses on a cool surface.
B)Helium gas escapes from a hole in a balloon.
C)Calcium carbonate stalactites form in a cave.
D)Water freezes in a freezer.
E)All of these increase entropy of the system.
A)Water vapor condenses on a cool surface.
B)Helium gas escapes from a hole in a balloon.
C)Calcium carbonate stalactites form in a cave.
D)Water freezes in a freezer.
E)All of these increase entropy of the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
61
If 3.50 g of Ni are reacted with excess oxygen to form nickel oxide (NiO)under standard state conditions,what is the entropy change for the reaction? 2 Ni(s)+ O2(g) 2 NiO(s)
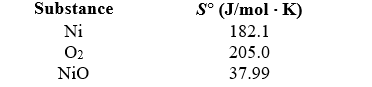
A)-49.3 J/ K
B)-24.7 J/ K
C)-14.7 J/ K
D)+49.3 J/ K
E)-10.4 J/ K
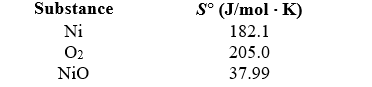
A)-49.3 J/ K
B)-24.7 J/ K
C)-14.7 J/ K
D)+49.3 J/ K
E)-10.4 J/ K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
62
Indicate which one of the following reactions result in a positive Ssys.
A)AgNO3(aq)+ NaCl(aq) AgCl(s)+ NaNO3(aq)
B)HCl(g)+ H2O( ) HCl(aq)
) HCl(aq)
C)H2(g)+ I2(g) 2HI(g)
D)C2H2O2(g) 2 CO(g)+ H2(g)
E)H2O(g) H2O( )
)
A)AgNO3(aq)+ NaCl(aq) AgCl(s)+ NaNO3(aq)
B)HCl(g)+ H2O(
 ) HCl(aq)
) HCl(aq)C)H2(g)+ I2(g) 2HI(g)
D)C2H2O2(g) 2 CO(g)+ H2(g)
E)H2O(g) H2O(
 )
)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
63
NO gas is converted to NO2 gas according to the following reaction: NO(g)+ 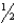 O2(g) NO2(g).What is the standard entropy change when 0.500 mol of NO gas reacts with 0.500 mol of O2 gas?
O2(g) NO2(g).What is the standard entropy change when 0.500 mol of NO gas reacts with 0.500 mol of O2 gas?

A)+36.6 J/K
B)-176 J/K
C)-83.4 J/K
D)+83.4 J/K
E)-36.6 J/K
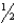 O2(g) NO2(g).What is the standard entropy change when 0.500 mol of NO gas reacts with 0.500 mol of O2 gas?
O2(g) NO2(g).What is the standard entropy change when 0.500 mol of NO gas reacts with 0.500 mol of O2 gas? 
A)+36.6 J/K
B)-176 J/K
C)-83.4 J/K
D)+83.4 J/K
E)-36.6 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following statements regarding free energy is NOT true?
A)Free energy represents the maximum work that a thermodynamic system can perform during a spontaneous change.
B)Free energy represents the minimum work that would be required to make a thermodynamic system undergo a nonspontaneous change.
C)The free energy of a system increases during the course of a spontaneous reaction as the entropy of the universe increases.
D)The Gibbs free energy change of a reaction reflects the work associated with processes happening at constant temperature and pressure.
E)The Gibbs free energy of a spontaneous process is negative.
A)Free energy represents the maximum work that a thermodynamic system can perform during a spontaneous change.
B)Free energy represents the minimum work that would be required to make a thermodynamic system undergo a nonspontaneous change.
C)The free energy of a system increases during the course of a spontaneous reaction as the entropy of the universe increases.
D)The Gibbs free energy change of a reaction reflects the work associated with processes happening at constant temperature and pressure.
E)The Gibbs free energy of a spontaneous process is negative.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
65
Indicate which one of the following reactions results in a negative Ssys.
A)H2O(g) H2O(s)
B)CaCO3(s) CaO(s)+ CO2(g)
C)CuSO4 5 H2O(s) CuSO4(s)+ 5 H2O(g)
D)14 O2(g)+ 3 NH4NO3(s)+ C10H22 3 N2(g)+ 17 H2O(g)+ 10 CO2(g)
3 N2(g)+ 17 H2O(g)+ 10 CO2(g)
E)CO2(aq) CO2(g)
A)H2O(g) H2O(s)
B)CaCO3(s) CaO(s)+ CO2(g)
C)CuSO4 5 H2O(s) CuSO4(s)+ 5 H2O(g)
D)14 O2(g)+ 3 NH4NO3(s)+ C10H22
 3 N2(g)+ 17 H2O(g)+ 10 CO2(g)
3 N2(g)+ 17 H2O(g)+ 10 CO2(g)E)CO2(aq) CO2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
66
Determine the standard entropy change of the universe for the reaction, NH4+(aq)+ NO3-(aq) NH4NO3(s),given the following information.Is the reaction spontaneous under standard conditions?

A)"-14.4 J/K,no"
B)"+28.1 J/K,yes"
C)"-94.3 J/K,no"
D)"+108.7 J/K,yes"
E)"+14.5 J/K,yes"

A)"-14.4 J/K,no"
B)"+28.1 J/K,yes"
C)"-94.3 J/K,no"
D)"+108.7 J/K,yes"
E)"+14.5 J/K,yes"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
67
In an experiment,1.000 atm of H2(g)in a 10.00 L container at 25.00 C was reacted under standard state conditions with a stoichiometric quantity of O2(g)to form water vapor.Determine the entropy change.

A)-146.5 J/K
B)-44.3 J/K
C)-18.1 J/K
D)+18.1 J/K
E)+44.3 J/K

A)-146.5 J/K
B)-44.3 J/K
C)-18.1 J/K
D)+18.1 J/K
E)+44.3 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
68
Determine the change in the standard entropy of the universe for the reaction,H2(g)+ 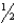 O2(g) H2O(g),given the following information:
O2(g) H2O(g),given the following information:

A)-766.6 J/K
B)+197.4 J/K
C)-197.4 J/K
D)+766.6 J/K
E)-228.6 J/K
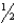 O2(g) H2O(g),given the following information:
O2(g) H2O(g),given the following information:
A)-766.6 J/K
B)+197.4 J/K
C)-197.4 J/K
D)+766.6 J/K
E)-228.6 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the standard entropy change when 10.0 g of methane reacts with 10.0 g of oxygen to form carbon dioxide and liquid water?
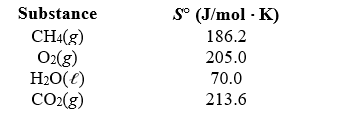
A)-121 J/K
B)-37.9 J/K
C)-243 J/K
D)-154 J/K
E)-16.8 J/K
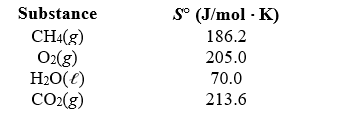
A)-121 J/K
B)-37.9 J/K
C)-243 J/K
D)-154 J/K
E)-16.8 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
70
Determine S for the reaction,N2O4(g) 2 NO2(g),given the following information.
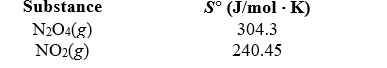
A)+176.6 J/mol . K
B)-63.8 J/mol . K
C)+63.8 J/mol . K
D)-50.7 J/mol . K
E)-176.7 J/mol . K
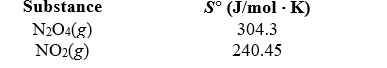
A)+176.6 J/mol . K
B)-63.8 J/mol . K
C)+63.8 J/mol . K
D)-50.7 J/mol . K
E)-176.7 J/mol . K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
71
Determine the entropy change for the reaction,SO2(g)+ 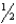 O2(g) SO3(g),given the following:
O2(g) SO3(g),given the following: 
A)-196.4 J/K
B)+196.4 J/K
C)-93.9 J/K
D)+93.9 J/K
E)+401.4 J/K
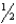 O2(g) SO3(g),given the following:
O2(g) SO3(g),given the following: 
A)-196.4 J/K
B)+196.4 J/K
C)-93.9 J/K
D)+93.9 J/K
E)+401.4 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the relationships between the free energy change of a system and associated entropy changes is true?
A)" Gsys = +T Ssys"
B)" Gsys = -T Ssys"
C)" Gsys = +T Suniv"
D)" Gsys = -T Ssurr"
E)" Gsys = -T Suniv'
A)" Gsys = +T Ssys"
B)" Gsys = -T Ssys"
C)" Gsys = +T Suniv"
D)" Gsys = -T Ssurr"
E)" Gsys = -T Suniv'

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
73
What is the entropy change if 4.50 g of CaCO3(s)is placed in a container and allowed to decompose to CaO(s)and CO2(g)according to the following reaction? CaCO3(s) CaO(s)+ CO2(g)

A)+7.22 J/K
B)-161 J/K
C)+35.7 J/K
D)+161 J/K
E)+3.57 J/K

A)+7.22 J/K
B)-161 J/K
C)+35.7 J/K
D)+161 J/K
E)+3.57 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
74
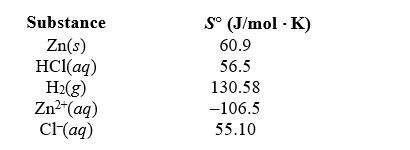
Determine S for the reaction,Zn(s)+ 2 HCl(aq) ZnCl2(aq)+ H2(g),given
A)0 J/K
B)-39.6 J/K
C)+39.6 J/K
D)-38.2 J/K
E)+38.2 J/K

A)0 J/K
B)-39.6 J/K
C)+39.6 J/K
D)-38.2 J/K
E)+38.2 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
75
Estimate the standard molar entropy of 1.00 M aqueous Mg2+ using the following information: S for the reaction,Mg(s)+ 2 HCl(aq) MgCl2(aq)+ H2(g),is -43.02 J/K.

A)"-52.1 J/K"
B)"+140.J/K"
C)"+138 J/K"
D)"-138 J/K"
E)"-140.J/K"

A)"-52.1 J/K"
B)"+140.J/K"
C)"+138 J/K"
D)"-138 J/K"
E)"-140.J/K"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
76
Determine S for the reaction,H2(g)+ I2(g) 2 HI(g),given the following information.

A)-41.1 J/mol . K
B)-165.3 J/mol . K
C)+398.8 J/mol . K
D)+165.3 J/mol . K
E)+41.1 J/mol . K

A)-41.1 J/mol . K
B)-165.3 J/mol . K
C)+398.8 J/mol . K
D)+165.3 J/mol . K
E)+41.1 J/mol . K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
77
The enthalpy and entropy of vaporization of acetone at its normal boiling point,-56.4 C,are 31.3 kJ/mol and 95.0 J/mol . K,respectively.What would the entropy change of the universe be if one mole of acetone vapor were to condense in a large room maintained at 25.0 C?
A)-2.0 102 J/K
B)+10.0 J/K
C)+1.05 102 J/K
D)-10.0 J/K
E)+2.0 102 J/K
A)-2.0 102 J/K
B)+10.0 J/K
C)+1.05 102 J/K
D)-10.0 J/K
E)+2.0 102 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
78
The enthalpy and entropy of vaporization of ammonia at its normal boiling point,-33.34 C,are 23.35 kJ/mol and 97.4 J/mol . K,respectively.What is the entropy change of the universe when one mole of liquid ammonia vaporizes in a large room maintained at 34.0 C? Assume the final temperature of the ammonia gas is -33.34 C.
A)+21.4 J/K
B)-76.0 J/K
C)+74.1 J/K
D)-21.4 J/K
E)+76.0 J/K
A)+21.4 J/K
B)-76.0 J/K
C)+74.1 J/K
D)-21.4 J/K
E)+76.0 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
79
In an experiment,1.000 mol of sodium metal is placed in a container and reacted with 4.000 mol of chlorine gas to form sodium chloride under standard state conditions.Determine Srxn given the following.
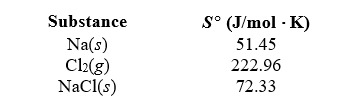
A)-181.2 J/K
B)-90.60 J/ K
C)-724.8 J/K
D)-45.30 J/K
E)-202.1 J/K
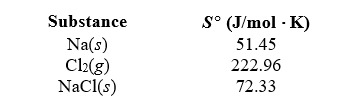
A)-181.2 J/K
B)-90.60 J/ K
C)-724.8 J/K
D)-45.30 J/K
E)-202.1 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck
80
The enthalpy and entropy of vaporization of ammonia at its normal boiling point,-33.34 C,are approximately 23.35 kJ/mol and 97.4 J/mol . K,respectively.What would the entropy change of the universe be if one mole of ammonia vapor were to condense in a large room maintained at 30.0 C?
A)"+20.4 J/K"
B)"+77.0 J/K"
C)"-174.4 J/K"
D)"-20.4 J/K"
E)"+174.4 J/K"
A)"+20.4 J/K"
B)"+77.0 J/K"
C)"-174.4 J/K"
D)"-20.4 J/K"
E)"+174.4 J/K"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 152 في هذه المجموعة.
فتح الحزمة
k this deck








