Deck 7: Stoichiometry-Mass Relationships and Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/149
العب
ملء الشاشة (f)
Deck 7: Stoichiometry-Mass Relationships and Chemical Reactions
1
One mole is defined as __________
A)the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B)the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C)the number of particles equal to the number of molecules in 1kg of water.
D)the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E)the number of particles equal to the number of atoms in exactly 1 kg of carbon at the National Bureau of Standards.
A)the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B)the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C)the number of particles equal to the number of molecules in 1kg of water.
D)the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E)the number of particles equal to the number of atoms in exactly 1 kg of carbon at the National Bureau of Standards.
the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
2
Which statement about a balanced chemical reaction equation is always correct?
A)The total number of moles of the products equals the total number of moles of the reactants.
B)The number of atoms of each kind is the same for the products as for the reactants.
C)The sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)The sum of the masses of all gaseous products equals the sum of the masses of the gaseous reactants.
E)The sum of the masses of solid products equals the sum of the masses of solid reactants.
A)The total number of moles of the products equals the total number of moles of the reactants.
B)The number of atoms of each kind is the same for the products as for the reactants.
C)The sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)The sum of the masses of all gaseous products equals the sum of the masses of the gaseous reactants.
E)The sum of the masses of solid products equals the sum of the masses of solid reactants.
The number of atoms of each kind is the same for the products as for the reactants.
3
Hydrogen peroxide decomposes to produce water and oxygen.Which relationship regarding the quantities of reactants and products associated with this reaction is NOT correct? 2 H2O2(  ) 2 H2O(
) 2 H2O(  )+ O2(g)
)+ O2(g)
A)2 molecules 2 molecules + 1 molecule
B)2 mol 2 mol + 1 mol
C)68.0 g 36.0 g + 32.0 g
D)2x mol 2x mol + x mol
E)y(34.0 g) y(18.0 g)+ y(32 g)
 ) 2 H2O(
) 2 H2O(  )+ O2(g)
)+ O2(g)A)2 molecules 2 molecules + 1 molecule
B)2 mol 2 mol + 1 mol
C)68.0 g 36.0 g + 32.0 g
D)2x mol 2x mol + x mol
E)y(34.0 g) y(18.0 g)+ y(32 g)
y(34.0 g) y(18.0 g)+ y(32 g)
4
Which of the following statements is NOT correct?
A)In the reaction,3 NO2 + H2O 2 HNO3 + xO,x must be a nitrogen atom.
B)If 100.00 grams of C2H4 react with 7.20 grams of H2 to form C2H6,the final mass of the product must be 107.20 grams.
C)If 32 grams of oxygen gas react with hydrogen to form water,the mass of the product must be 32 grams.
D)When 2 moles of HNO3 form from the complete reaction of N2O5 and H2O,108 grams of N2O5 must have been used.
E)The mass of CO2 produced by the reaction CH4 + 2 O2 CO2 + 2 H2O equals the sum of the initial masses of CH4 and O2 minus the mass of water formed.
A)In the reaction,3 NO2 + H2O 2 HNO3 + xO,x must be a nitrogen atom.
B)If 100.00 grams of C2H4 react with 7.20 grams of H2 to form C2H6,the final mass of the product must be 107.20 grams.
C)If 32 grams of oxygen gas react with hydrogen to form water,the mass of the product must be 32 grams.
D)When 2 moles of HNO3 form from the complete reaction of N2O5 and H2O,108 grams of N2O5 must have been used.
E)The mass of CO2 produced by the reaction CH4 + 2 O2 CO2 + 2 H2O equals the sum of the initial masses of CH4 and O2 minus the mass of water formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the molar mass of phosphorus pentachloride (PCl5)?
A)177.3 g/mol
B)190.3 g/mol
C)208.2 g/mol
D)172.8 g/mol
E)202.8 g/mol
A)177.3 g/mol
B)190.3 g/mol
C)208.2 g/mol
D)172.8 g/mol
E)202.8 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
6
How many moles of chlorine are in 25.0 g of calcium chloride (CaCl2)?
A)0.705 mol
B)0.451 mol
C)0.331 mol
D)0.225 mol
E)0.676 mol
A)0.705 mol
B)0.451 mol
C)0.331 mol
D)0.225 mol
E)0.676 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which relationship regarding the quantities of reactants and products associated with the following reaction is NOT correct? 6 H2O(  )+ P4O10 (s) 4 H3PO(aq)
)+ P4O10 (s) 4 H3PO(aq)
A)6 molecules + 1 molecule 4 molecules
B)18 mol + 6 mol 12 mol
C)324 g + 852 g 1176 g
D)6x mol + x mol 4x mol
E)y(27 g)+ y(71 g) y(98 g)
 )+ P4O10 (s) 4 H3PO(aq)
)+ P4O10 (s) 4 H3PO(aq)A)6 molecules + 1 molecule 4 molecules
B)18 mol + 6 mol 12 mol
C)324 g + 852 g 1176 g
D)6x mol + x mol 4x mol
E)y(27 g)+ y(71 g) y(98 g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following contains the largest number of atoms?
A)1 mol water
B)1 mol phosphorus trichloride
C)1 mol dinitrogen pentoxide
D)2 mol carbon monoxide
E)All of these contain the same number of atoms.
A)1 mol water
B)1 mol phosphorus trichloride
C)1 mol dinitrogen pentoxide
D)2 mol carbon monoxide
E)All of these contain the same number of atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
9
A gallon of water has a mass of 3.79 kg.How many moles of water is this?
A)210 mol
B)0.210 mol
C)68.3 mol
D)68,300 mol
E)386 mol
A)210 mol
B)0.210 mol
C)68.3 mol
D)68,300 mol
E)386 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many moles of ammonia are there in a 346 g sample of pure NH3 (17.03 g/mol)?
A)0.0496 mol
B)20.3 mol
C)24.7 mol
D)5930 mol
E)3.46 mol
A)0.0496 mol
B)20.3 mol
C)24.7 mol
D)5930 mol
E)3.46 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
11
Fuming sulfuric acid is obtained by the addition of SO3 to concentrated H2SO4.The fumes result from the reaction of SO3 gas with water vapor.What is the product when one molecule of SO3 reacts with one molecule of water?
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)two molecules of sulfuric acid
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)two molecules of sulfuric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which statement about the following chemical reaction is NOT correct? 3 H2(g)+ N2(g) 2 NH3(g)
A)For every nitrogen molecule consumed,two molecules of ammonia are produced.
B)For every 6.06 grams of H2 consumed,2 moles of NH3 are produced.
C)If 34.0 grams of NH3 are produced,28.0 grams of N2 were consumed.
D)In order for 56 grams of N2 to completely react,18 grams of H2 would be required.
E)Three moles of nitrogen react with nine moles of hydrogen.
A)For every nitrogen molecule consumed,two molecules of ammonia are produced.
B)For every 6.06 grams of H2 consumed,2 moles of NH3 are produced.
C)If 34.0 grams of NH3 are produced,28.0 grams of N2 were consumed.
D)In order for 56 grams of N2 to completely react,18 grams of H2 would be required.
E)Three moles of nitrogen react with nine moles of hydrogen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the molar mass of sulfuric acid (H2SO4)?
A)49.0 g/mol
B)24.5 g/mol
C)101 g/mol
D)98.1 g/mol
E)97.0 g/mol
A)49.0 g/mol
B)24.5 g/mol
C)101 g/mol
D)98.1 g/mol
E)97.0 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which statement about the following chemical reaction is NOT correct? 3 H2(g)+ N2(g) 2 NH3(g)
A)For every two moles of nitrogen consumed,three moles of ammonia are produced.
B)For every mole of nitrogen consumed,two moles of ammonia are produced.
C)For every three moles of hydrogen consumed,two moles of ammonia are produced.
D)For every two moles of ammonia produced,three moles of hydrogen are consumed.
E)Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.
A)For every two moles of nitrogen consumed,three moles of ammonia are produced.
B)For every mole of nitrogen consumed,two moles of ammonia are produced.
C)For every three moles of hydrogen consumed,two moles of ammonia are produced.
D)For every two moles of ammonia produced,three moles of hydrogen are consumed.
E)Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
15
Hydrogen peroxide decomposes to produce water and oxygen.Which relationship regarding the quantities of reactants and products associated with this reaction is NOT correct? 2 H2O2
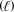 2 H2O
2 H2O 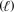 + O2(g)
+ O2(g)
A)68.0 g 36.0 g + 32.0 g
B)34.0 g 18.0 g + 16.0 g
C)90.4 g 47.9 g + 42.6 g
D)2x g 2x g + x g
E)y(34.0 g) y(18.0 g)+ (y/2)(32 g)
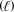 2 H2O
2 H2O 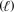 + O2(g)
+ O2(g)A)68.0 g 36.0 g + 32.0 g
B)34.0 g 18.0 g + 16.0 g
C)90.4 g 47.9 g + 42.6 g
D)2x g 2x g + x g
E)y(34.0 g) y(18.0 g)+ (y/2)(32 g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which statement about the following chemical reaction is NOT correct? The molar masses of S8,O2,and SO3 are 256,32.0,and 80.1 g/mol,respectively. S8(s)+ 12 O2(g) 8 SO3(g)
A)1 mol S8 produces 8 mol SO3.
B)256 g S8 requires 12 mol O2 in order to completely react.
C)In order to produce 8 mol SO3,256 g S8 and 192 g O2 must react.
D)For every 6 mol O2 consumed,4 mol SO3 are produced.
E)If 641 g SO3 are produced,a total mass of 641 g of S8 and O2 must react.
A)1 mol S8 produces 8 mol SO3.
B)256 g S8 requires 12 mol O2 in order to completely react.
C)In order to produce 8 mol SO3,256 g S8 and 192 g O2 must react.
D)For every 6 mol O2 consumed,4 mol SO3 are produced.
E)If 641 g SO3 are produced,a total mass of 641 g of S8 and O2 must react.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
17
A sample of water (H2O)contains 1.81 1024 molecules.How many moles of atoms are there in this sample?
A)1.00
B)2.00
C)3.00
D)6.00
E)9.00
A)1.00
B)2.00
C)3.00
D)6.00
E)9.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which one of the following samples contains the fewest number of molecules? H is used as the symbol for hydrogen-1,and D is used as the symbol for hydrogen-2.
A)42 g of D2O
B)2 moles of H2O
C)2 moles of D2O
D)36 g of H2O
E)39 g of D2O
A)42 g of D2O
B)2 moles of H2O
C)2 moles of D2O
D)36 g of H2O
E)39 g of D2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
19
Fe2O3(s)and powdered aluminum can react with great output of heat to form molten iron and Al2O3.When this reaction equation is balanced,what are the stoichiometric coefficients in the following order: Fe2O3,Al,Fe,Al2O3?
A)1,1,1,1
B)2,2,2,2
C)1,2,2,1
D)2,1,1,2
E)1,1,2,2
A)1,1,1,1
B)2,2,2,2
C)1,2,2,1
D)2,1,1,2
E)1,1,2,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
20
Hydrogen peroxide decomposes to produce water and oxygen.Which of the following statements is NOT correct? 2 H2O2(  ) 2 H2O(
) 2 H2O(  )+ O2(g)
)+ O2(g)
A)The products of the reaction are liquids.
B)Water and oxygen are the products of the reaction.
C)The chemical formula of hydrogen peroxide is H2O2.
D)The reaction is balanced.
E)Two molecules of H2O and one molecule of O2 are produced when two molecules of H2O2 react.
 ) 2 H2O(
) 2 H2O(  )+ O2(g)
)+ O2(g)A)The products of the reaction are liquids.
B)Water and oxygen are the products of the reaction.
C)The chemical formula of hydrogen peroxide is H2O2.
D)The reaction is balanced.
E)Two molecules of H2O and one molecule of O2 are produced when two molecules of H2O2 react.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
21
The average adult exhales about 1.0 kg of carbon dioxide each day.How much oxygen is needed in metabolizing glucose (C6H12O6,180 g/mol)to make that much carbon dioxide?
A)180 g
B)1800 g
C)360 g
D)730 g
E)1500 g
A)180 g
B)1800 g
C)360 g
D)730 g
E)1500 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
22
The combustion of butane (C4H10)forms carbon dioxide and water.What is the stoichiometric coefficient for oxygen in the balanced equation when 1 mol of butane undergoes combustion?
A)9
B)13
C)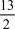
D)
E)5
A)9
B)13
C)
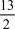
D)

E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
23
During a fermentation process,sucrose (C12H22O11)can react with water to form ethanol (C2H5OH)and carbon dioxide gas.What is the sum of the coefficients in the balanced chemical equation?
A)8
B)10
C)12
D)14
E)16
A)8
B)10
C)12
D)14
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
24
Identify the set of stoichiometric coefficients that balance the reaction equation for the combustion of octane. C8H18
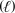 + O2(g) CO2(g)+ H2O
+ O2(g) CO2(g)+ H2O 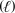
A)1,25,8,9
B)1,17,8,9
C)2,34,16,18
D)2,25,16,18
E)None of the above coefficients balance the reaction equation
 + O2(g) CO2(g)+ H2O
+ O2(g) CO2(g)+ H2O 
A)1,25,8,9
B)1,17,8,9
C)2,34,16,18
D)2,25,16,18
E)None of the above coefficients balance the reaction equation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
25
In a one type of fuel cell,methanol (CH3OH)enters on one side of the unit,and air enters on the other side.Both circulate past electrodes and chemically react to produce carbon dioxide and water along with electricity.Which chemical equation best represents the reaction that occurs?
A)CH3OH CO2(g)+ 2 H2O
CO2(g)+ 2 H2O 
B)2 CH3OH + 3 O2(g) 2 CO2(g)+ 4H2O
+ 3 O2(g) 2 CO2(g)+ 4H2O 
C)CH3OH CH2(g)+ H2O
CH2(g)+ H2O 
D)CH3OH + N2(g) CH3(g)+ NO(g)
+ N2(g) CH3(g)+ NO(g)
E)CH3OH + O2(g) CO2(g)+ H2O
+ O2(g) CO2(g)+ H2O 
A)CH3OH
 CO2(g)+ 2 H2O
CO2(g)+ 2 H2O 
B)2 CH3OH
 + 3 O2(g) 2 CO2(g)+ 4H2O
+ 3 O2(g) 2 CO2(g)+ 4H2O 
C)CH3OH
 CH2(g)+ H2O
CH2(g)+ H2O 
D)CH3OH
 + N2(g) CH3(g)+ NO(g)
+ N2(g) CH3(g)+ NO(g)E)CH3OH
 + O2(g) CO2(g)+ H2O
+ O2(g) CO2(g)+ H2O 

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
26
Sulfuric acid reacts with a vanadium oxide compound according to the following unbalanced reaction.What are the coefficients in the balanced chemical equation in the order in which it is written? H2SO4(aq)+ V2O3(s) V2(SO4)3(s)+ H2O 
A)3,1,1,3
B)3,2,3,3
C)3,1,1,6
D)3,2,2,6
E)6,3,3,12
A)3,1,1,3
B)3,2,3,3
C)3,1,1,6
D)3,2,2,6
E)6,3,3,12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
27
Extracting copper from sulfide ores can be performed by roasting the ore in oxygen,with a subsequent reaction of copper (I)oxide with carbon.Simplified chemical equations for these steps are shown below.Mathematically combine the two reactions to create an overall balanced equation.Eliminate like terms from your final equation and use the lowest whole-number coefficients possible.Select the statement regarding the overall balanced reaction that is NOT true. 2 Cu2S(s)+ 3 O2(g) 2 Cu2O(s)+ 2 SO2(g)
Cu2O(s)+ C(s) 2 Cu(s)+ CO(g)
A)The balanced reaction requires three moles of oxygen gas.
B)Two moles of copper result when two moles of carbon react.
C)Cu2O does not appear in the final balanced equation.
D)Two moles of CO gas are produced in the overall balanced equation.
E)There are three reactants and three products in the overall balanced equation.
Cu2O(s)+ C(s) 2 Cu(s)+ CO(g)
A)The balanced reaction requires three moles of oxygen gas.
B)Two moles of copper result when two moles of carbon react.
C)Cu2O does not appear in the final balanced equation.
D)Two moles of CO gas are produced in the overall balanced equation.
E)There are three reactants and three products in the overall balanced equation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
28
Air bags in cars inflate when an electrical spark activates sodium azide (NaN3),so that it decomposes to sodium metal and nitrogen gas.In the balanced reaction equation,how many moles of N2 gas are formed for each mole of NaN3?
A)1
B)1.5
C)2
D)3
E)2.5
A)1
B)1.5
C)2
D)3
E)2.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which statement,A-D,regarding photosynthesis is NOT correct? In the process of photosynthesis,__________
A)light energy is converted into carbon-based fuels.
B)oxygen is produced.
C)atmospheric carbon dioxide is consumed.
D)the sugar glucose is produced.
E)A-D are all correct.
A)light energy is converted into carbon-based fuels.
B)oxygen is produced.
C)atmospheric carbon dioxide is consumed.
D)the sugar glucose is produced.
E)A-D are all correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
30
Chlorine atoms in the atmosphere can react with ozone.Mathematically combine the following two reactions to create an overall balanced equation with the lowest whole-number coefficients and select the statement that is NOT true.All chemical species in the reaction are gas-phase. Cl + O3 ClO + O2
ClO + O Cl + O2
A)Chlorine atoms do not appear in the overall balanced reaction.
B)There is a net conversion of O3 to O2.
C)ClO does not appear in the overall balanced reaction.
D)Two chlorine atoms can cause the production of two oxygen atoms.
E)Oxygen atoms are produced by the overall reaction.
ClO + O Cl + O2
A)Chlorine atoms do not appear in the overall balanced reaction.
B)There is a net conversion of O3 to O2.
C)ClO does not appear in the overall balanced reaction.
D)Two chlorine atoms can cause the production of two oxygen atoms.
E)Oxygen atoms are produced by the overall reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
31
Potassium perchlorate (KClO3)oxidizes sucrose (C12H22O11)according to the following unbalanced reaction.What are the coefficients in the balanced chemical equation in the order in which it is written? KClO3(s)+ C12H22O11(s) KCl(s)+ CO2(g)+ H2O 
A)4,1,4,12,11
B)4,1,4,12,22
C)2,1,2,12,11
D)8,1,8,12,11
E)8,2,8,12,11
A)4,1,4,12,11
B)4,1,4,12,22
C)2,1,2,12,11
D)8,1,8,12,11
E)8,2,8,12,11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
32
Diesel fuel for automobiles and trucks is a mixture of hydrocarbons that can be modeled by C16H34.Write the balanced reaction equation for the combustion of C16H34,and report the sum of the stoichiometric coefficients.
A)117
B)120
C)76
D)83
E)4
A)117
B)120
C)76
D)83
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
33
Glucose (C6H12O6)is oxidized by molecular oxygen to carbon dioxide and water.How many O2 molecules are needed for each molecule of glucose that is oxidized?
A)1
B)2
C)6
D)12
E)18
A)1
B)2
C)6
D)12
E)18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
34
The acid-base reaction between phosphoric acid,H3PO4,and calcium hydroxide,Ca(OH)2,yields water and calcium phosphate.For each mole of calcium phosphate produced by this reaction,how many moles of water are produced?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
35
Sulfur dioxide from coal-fired power plants combines with water in the atmosphere to produce acid rain.What is the product when one molecule of SO2 reacts with one molecule of water?
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)one molecule of sulfurous acid
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)one molecule of sulfurous acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
36
What are the stoichiometric coefficients for oxygen and water,respectively,in the balanced chemical reaction equation representing the combustion of butane (C4H10)?
A)13,10
B)9,5
C)4,5
D) ,10
,10
E)13,5
A)13,10
B)9,5
C)4,5
D)
 ,10
,10E)13,5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
37
Hydrazine (N2H4)can decompose to form nitrogen gas and ammonia gas.What is the sum of the coefficients in the balanced chemical equation?
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
38
In a demonstration of the complete combustion of propane (C3H8)with oxygen (O2),several balloons were prepared with various proportions by volume of propane and oxygen.The loudest explosion occurred for the balloon with the correct stoichiometric proportions of the gases.Which balloon had the loudest explosion?
A)1 portion propane to 1 portion oxygen
B)1 portion propane to 3 portions oxygen
C)1 portion propane to 4 portions oxygen
D)1 portion propane to 5 portions oxygen
E)2 portions propane to 3 portions oxygen
A)1 portion propane to 1 portion oxygen
B)1 portion propane to 3 portions oxygen
C)1 portion propane to 4 portions oxygen
D)1 portion propane to 5 portions oxygen
E)2 portions propane to 3 portions oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
39
In an aqueous solution under basic conditions,ozone (O3)reacts with iodide (I-)and water to form iodine (I2),hydroxide (OH-),and oxygen (O2).What is the sum of the stoichiometric coefficients in the balanced reaction equation? O3(g)+ I-(aq)+ H2O  I2(aq)+ OH-(aq)+ O2(g)
I2(aq)+ OH-(aq)+ O2(g)
A)6
B)8
C)10
D)12
E)14
 I2(aq)+ OH-(aq)+ O2(g)
I2(aq)+ OH-(aq)+ O2(g)A)6
B)8
C)10
D)12
E)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
40
One form of elemental sulfur is a ring of eight sulfur atoms.How many moles of molecular oxygen are consumed when one mole of this allotrope burns to make sulfur trioxide?
A)3
B)6
C)12
D)18
E)24
A)3
B)6
C)12
D)18
E)24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
41
The space shuttle uses liquid hydrogen and liquid oxygen to produce thrust for liftoff in the following reaction: 2 H2(l)+ O2(  ) 2 H2O(g)
) 2 H2O(g)
If the liquid oxygen supply is set to provide 420 kg/s during a launch,what does the rate of liquid hydrogen supply need to be in kilograms per second?
A)840 kg/s
B)210 kg/s
C)106 kg/s
D)52.9 kg/s
E)63.4 kg/s
 ) 2 H2O(g)
) 2 H2O(g)If the liquid oxygen supply is set to provide 420 kg/s during a launch,what does the rate of liquid hydrogen supply need to be in kilograms per second?
A)840 kg/s
B)210 kg/s
C)106 kg/s
D)52.9 kg/s
E)63.4 kg/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
42
The combustion of ethanol (CH3CH2OH,46.1 g/mol)results in the formation of water and carbon dioxide.How many grams of carbon dioxide are produced when 46.1 g of ethanol burns?
A)88.0 g
B)44.0 g
C)176 g
D)22.0 g
E)11.0 g
A)88.0 g
B)44.0 g
C)176 g
D)22.0 g
E)11.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
43
The most abundant metal in Earth's crust is aluminum,found mostly in the form of clays.Bauxite ore,impure hydrated aluminum oxide,can be purified and refined to make the metal.After the first purification step,hydrated aluminum oxide (Al2O3 . xH2O)is obtained.When 100.0 g of this solid was heated and the water driven off,65.36 g of Al2O3 remained.How many water molecules (x in the molecular formula)were there in the hydrate?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
44
As ammonium hydrogen carbonate (NH4HCO3,79.1 g/mol)is heated,it breaks down into three gases: ammonia,water,and carbon dioxide.For each 20.0 g of ammonium hydrogen carbonate heated (about 4 tsp),how many grams of carbon dioxide are produced?
A)15.9 g
B)6.67 g
C)5.06 g
D)11.1 g
E)20.0 g
A)15.9 g
B)6.67 g
C)5.06 g
D)11.1 g
E)20.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
45
Suppose the combustion of fossil fuels adds about 8.0 1012 kg of carbon to the atmosphere per year in the form of CO2.How much oxygen gas is required to produce this amount of carbon dioxide?
A)about 2.1 1013 kg
B)about 5.8 1012 kg
C)about 4.3 1012 kg
D)about 1.1 1013 kg
E)about 4.8 1013 kg
A)about 2.1 1013 kg
B)about 5.8 1012 kg
C)about 4.3 1012 kg
D)about 1.1 1013 kg
E)about 4.8 1013 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which statement,A-D,regarding the carbon cycle is NOT correct?
A)Petroleum,coal,and other carbon sediments are produced.
B)Respiration produces atmospheric carbon dioxide.
C)Water is a reactant in photosynthesis.
D)Forest fires are part of the carbon cycle.
E)A-D are all correct.
A)Petroleum,coal,and other carbon sediments are produced.
B)Respiration produces atmospheric carbon dioxide.
C)Water is a reactant in photosynthesis.
D)Forest fires are part of the carbon cycle.
E)A-D are all correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
47
Hydrogen peroxide (H2O2)decomposes catalytically in the presence of metal ions and enzymes called peroxidases.If a 250 mL bottle of hydrogen peroxide solution containing 3.0% H2O2 (by mass)decomposes completely,how many liters of oxygen gas would it generate? Assume that 1 mol of gas occupies a volume of 22.4 L and that the density of the solution is 1.0 g/mL.The reaction is 2 H2O2(aq) 2 H2O(  )+ O2(g)
)+ O2(g)
A)2.5 L
B)4.9 L
C)9.9 L
D)7.4 L
E)0.22 L
 )+ O2(g)
)+ O2(g)A)2.5 L
B)4.9 L
C)9.9 L
D)7.4 L
E)0.22 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
48
Copper was the first metal to be produced from its ore because it is the easiest to smelt,that is,to refine by heating in the presence of carbon.The ore was likely malachite [Cu2(OH)2CO3].What is the mass percent of copper in malachite?
A)28.7%
B)45.2%
C)57.5%
D)40.3%
E)74.6%
A)28.7%
B)45.2%
C)57.5%
D)40.3%
E)74.6%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
49
The average adult exhales about 1.0 kg of carbon dioxide each day.How much glucose (C6H12O6, 180 g/mol)is consumed to make that much carbon dioxide?
A)3.8 kg
B)4.1 kg
C)0.43 kg
D)0.68 kg
E)0.18 kg
A)3.8 kg
B)4.1 kg
C)0.43 kg
D)0.68 kg
E)0.18 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
50
Assuming complete combustion,how many grams of butane (C4H10)would be required to consume 32.0 g O2?
A)1.82 g
B)16.6 g
C)8.30 g
D)4.47g
E)8.94 g
A)1.82 g
B)16.6 g
C)8.30 g
D)4.47g
E)8.94 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
51
How many grams of CO2 would result from the complete combustion of 4.00 mol butane (C4H10)?
A)24.2 g
B)176 g
C)704 g
D)96.9 g
E)1410 g
A)24.2 g
B)176 g
C)704 g
D)96.9 g
E)1410 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
52
Water can be separated into its elements according to the following equation by electrolysis.If 20.0 g of water is decomposed by this method,how much oxygen gas is produced? 2 H2O(  ) 2 H2(g)+ O2(g)
) 2 H2(g)+ O2(g)
A)10.0 g
B)8.90 g
C)35.6 g
D)16.0 g
E)17.8 g
 ) 2 H2(g)+ O2(g)
) 2 H2(g)+ O2(g)A)10.0 g
B)8.90 g
C)35.6 g
D)16.0 g
E)17.8 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
53
Diesel fuel for automobiles and trucks is a mixture of hydrocarbons that can be modeled by C16H34.How many mL of C16H34 are needed to react with 1.00 mol O2? The density of C16H34 is 0.773 g/mL and its molar mass is 226 g/mol.
A)9.23 mL
B)11.9 mL
C)0.561 mL
D)4.61 mL
E)0.140 mL
A)9.23 mL
B)11.9 mL
C)0.561 mL
D)4.61 mL
E)0.140 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
54
Aluminum can form clusters that behave similarly to certain atoms. The “superatom,” Al13, is thought to mimic chlorine. Suppose you create a process that generates KAl13 (389.86 g/mol) and oxygen gas from aluminum oxide (101.96 g/mol) and potassium metal. How many grams of KAl13 could be produced by the reaction of 102 grams of Al2O3? You will need to write a balanced chemical reaction.
A)390 g
B)97.5 g
C)60.0 g
D)15.00 g
E)141 g
A)390 g
B)97.5 g
C)60.0 g
D)15.00 g
E)141 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
55
How much CO2 is produced by a car driven 20,000 mi in one year? Assume the following:
• A car gets 20 mi/4.0 L of gasoline.
• Gasoline has the chemical formula of octane: C8H18.
• One liter of gasoline has a mass of 0.8 kg.
• Gasoline is completely burned to carbon dioxide and water.
A)1,500 kg
B)9,900 kg
C)15,000 kg
D)20,000 kg
E)4,900 kg
• A car gets 20 mi/4.0 L of gasoline.
• Gasoline has the chemical formula of octane: C8H18.
• One liter of gasoline has a mass of 0.8 kg.
• Gasoline is completely burned to carbon dioxide and water.
A)1,500 kg
B)9,900 kg
C)15,000 kg
D)20,000 kg
E)4,900 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
56
If 20.0 kg CO2 are produced from the combustion of butane (C4H10),calculate the mass of oxygen gas that was consumed.
A)23.6 kg
B)18.2 kg
C)11.8 kg
D)739 g
E)454 g
A)23.6 kg
B)18.2 kg
C)11.8 kg
D)739 g
E)454 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
57
Global carbon emissions in 2010 were estimated to be on the order of 9.0 1012 metric tons carbon (1 metric ton = 1000 kg)in the form of CO2.This is equivalent to __________ kg C.
A)about 2.5 1015 kg
B)about 7.5 1014 kg
C)about 2.0 1014 kg
D)about 1.7 1013 kg
E)about 1.2 1015 kg
A)about 2.5 1015 kg
B)about 7.5 1014 kg
C)about 2.0 1014 kg
D)about 1.7 1013 kg
E)about 1.2 1015 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
58
Copper(II)sulfate (CuSO4)can be used to control algae growth.Aqueous CuSO4 solutions that come in contact with the surface of galvanized (zinc-plated)steel pails undergo the following reaction: CuSO4(aq)+ Zn(s) Cu(s)+ ZnSO4(aq)
How many grams of zinc would react with 454 g (1 lb)of CuSO4 (160 g/mol)?
A)467 g
B)186 g
C)93 g
D)234 g
E)454 g
How many grams of zinc would react with 454 g (1 lb)of CuSO4 (160 g/mol)?
A)467 g
B)186 g
C)93 g
D)234 g
E)454 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
59
Diesel fuel for automobiles and trucks is a mixture of hydrocarbons that can be modeled by C16H34.How many grams of O2 are needed to react with 0.500 mol C16H34?
A)458 g
B)392 g
C)784 g
D)295g
E)196 g
A)458 g
B)392 g
C)784 g
D)295g
E)196 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
60
Arizona was the site of a 400,000-acre wildfire in June 2002.How much carbon dioxide was produced by this fire? Assume that the density of carbon on the acreage was 10 kg/m2 and that 50% of the biomass burned (10,000 m2 = 2.47 acre).
A)3 1010 kg
B)3 106 kg
C)3 1030 kg
D)3 109 kg
E)3 1013 kg
A)3 1010 kg
B)3 106 kg
C)3 1030 kg
D)3 109 kg
E)3 1013 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
61
A compound containing bromine and fluorine used in the production of UF6 is 58.37% Br by mass.What is the empirical formula for the compound?
A)BrF
B)Br2F3
C)BrF3
D)BrF5
E)Br3F2
A)BrF
B)Br2F3
C)BrF3
D)BrF5
E)Br3F2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
62
Ibuprofen is 75.69% C,8.80% H,and 15.51% O by mass.What is its empirical formula?
A)C6H9O
B)C9H9O2
C)C7H18O2
D)C7H9O2
E)C13H18O2
A)C6H9O
B)C9H9O2
C)C7H18O2
D)C7H9O2
E)C13H18O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
63
A compound known to contain platinum,nitrogen,and hydrogen was analyzed and found to contain 74.1% Pt and 21.3% N; the remainder was hydrogen.What is the empirical formula for the compound?
A)Pt(NH3)4
B)Pt(NH3)3
C)Pt2(NH3)2
D)Pt(NH4)2
E)Pt(NH3)6
A)Pt(NH3)4
B)Pt(NH3)3
C)Pt2(NH3)2
D)Pt(NH4)2
E)Pt(NH3)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following does NOT share the same empirical formula as the others?
A)formaldehyde,CH2O
B)acetic acid,CH3COOH
C)lactic acid,CH3CH(OH)COOH
D)butyric acid,CH3CH2CH2COOH
E)glucose,C6H12O6
A)formaldehyde,CH2O
B)acetic acid,CH3COOH
C)lactic acid,CH3CH(OH)COOH
D)butyric acid,CH3CH2CH2COOH
E)glucose,C6H12O6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
65
A 2.00 g sample of methamphetamine was found to contain 1.61 g C,0.203 g H,and 0.188 g N.What is its empirical formula?
A)C10H15N
B)C4H6N
C)C4H9N
D)C13H20N
E)C8H10N
A)C10H15N
B)C4H6N
C)C4H9N
D)C13H20N
E)C8H10N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
66
Alkanes share a general chemical formula of CnH2n+2.What is n of an alkane with a molar mass in the range of 125-130 g/mol?
A)10
B)9
C)8
D)11
E)12
A)10
B)9
C)8
D)11
E)12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
67
When are an empirical formula and a molecular formula the same?
A)Always
B)When the mass of the empirical formula unit is equal to the molecular mass
C)When the mass percentages of all of the elements in the two formulas are the same
D)Never
E)When the molecular mass is an integer multiple of formula unit mass
A)Always
B)When the mass of the empirical formula unit is equal to the molecular mass
C)When the mass percentages of all of the elements in the two formulas are the same
D)Never
E)When the molecular mass is an integer multiple of formula unit mass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
68
A hydrocarbon is found to be 85.63% C by mass.What is its empirical formula?
A)CH
B)CH2
C)CH3
D)C2H
E)CH4
A)CH
B)CH2
C)CH3
D)C2H
E)CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
69
Metallic copper can be obtained from the mineral chalcocite (Cu2S).What is the mass percent of copper in chalcocite?
A)79.85%
B)66.46%
C)20.15%
D)33.54%
E)57.47%
A)79.85%
B)66.46%
C)20.15%
D)33.54%
E)57.47%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
70
Paris green is a highly toxic emerald green compound with the formula Cu(C2H3O2)2 . 3Cu(AsO2)2.What is its percent by mass arsenic?
A)31.99%
B)14.78%
C)22.17%
D)7.389%
E)44.34%
A)31.99%
B)14.78%
C)22.17%
D)7.389%
E)44.34%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
71
Hydroxylamine contains 42.41% N and 9.15% H by mass,with the remainder being oxygen.Determine its empirical formula.
A)N14H3O16
B)N4.6HO5.3
C)N5HO5
D)N3H9O
E)NH3O
A)N14H3O16
B)N4.6HO5.3
C)N5HO5
D)N3H9O
E)NH3O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
72
The empirical formula for buckminsterfullerene is C1,and its molar mass is 720.6 g/mol.What is its molecular formula?
A)C60
B)C72
C)C12
D)C1
E)C720
A)C60
B)C72
C)C12
D)C1
E)C720

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
73
Putrescine,a foul-smelling compound produced by decaying tissue,has the empirical formula C2H6N,and its molecular mass is found to be between 87 and 90 amu.How many empirical units are in the actual molecular formula?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
74
An iron ore,magnetite,contains only iron and oxygen.In refining 100.0 g of the ore,72.35 g of iron are obtained.What is the empirical formula of the ore?
A)Fe2O3
B)FeO2
C)Fe2O5
D)Fe3O4
E)FeO
A)Fe2O3
B)FeO2
C)Fe2O5
D)Fe3O4
E)FeO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
75
A uranium oxide compound is found to be 88.15% U by mass.What is the empirical formula for the compound?
A)U2O3
B)UO3
C)U3O4
D)UO2
E)UO
A)U2O3
B)UO3
C)U3O4
D)UO2
E)UO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
76
Analysis of two uranium fluoride compounds yields the following data: Compound I is 75.80% U by mass; compound II has an F-to-U mass ratio that is 1.5 times that of compound I.What is the empirical formula for compound II?
A)UF4
B)UF6
C)U2F3
D)UF3
E)UF2
A)UF4
B)UF6
C)U2F3
D)UF3
E)UF2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
77
A gas that is produced when fruit ripens contains 85.62% C and 14.38% H by mass.The molecular mass was found to be twice the empirical formula unit molecular mass.What is the molecular formula of this gas?
A)CH2
B)C2H2
C)C2H4
D)C2H6
E)C4H4
A)CH2
B)C2H2
C)C2H4
D)C2H6
E)C4H4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
78
Han purple,a synthetic inorganic pigment developed in China in ancient times,has the chemical formula BaCuSi2O6.What is its percent by mass silicon?
A)18.0%
B)15.9%
C)27.2%
D)38.9%
E)7.96%
A)18.0%
B)15.9%
C)27.2%
D)38.9%
E)7.96%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
79
Caffeine has an elemental analysis of 49.48% C,5.190% H,16.47% O,and 28.85% N.It has a molar mass of 194.19 g/mol.What is the molecular formula of caffeine?
A)C4H5N2O
B)C8H10N4O2
C)C10H14N2O2
D)C6H6NO2
E)C5H7NO
A)C4H5N2O
B)C8H10N4O2
C)C10H14N2O2
D)C6H6NO2
E)C5H7NO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck
80
Phthalocyanine is a large molecule used in printing inks and dyes.Elemental analysis showed that it consists of 74.69% C,3.525% H,and 21.77 % N,and has a molar mass of 514.54 g/mol.What is its molecular formula?
A)C16H9N4
B)C30H8N10
C)C32H18N8
D)C32H4N9
E)C16H18N4
A)C16H9N4
B)C30H8N10
C)C32H18N8
D)C32H4N9
E)C16H18N4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 149 في هذه المجموعة.
فتح الحزمة
k this deck








