Deck 2: Atoms, ions, and Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/157
العب
ملء الشاشة (f)
Deck 2: Atoms, ions, and Compounds
1
What is the correct symbol for a - particle?
A)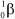
B)
C)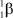
D)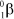
E)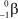
A)
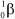
B)

C)
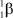
D)
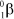
E)
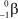
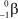
2
Which subatomic particles have opposite charges?
A)protons and neutrons
B)protons and electrons
C)neutrons and electrons
D)all protons
E)all neutrons
A)protons and neutrons
B)protons and electrons
C)neutrons and electrons
D)all protons
E)all neutrons
protons and electrons
3
A baseball has a diameter of approximately 7.4 cm (2.9 inches),whereas a carbon atom has a diameter of about 140 pm.How many times larger is the baseball than the carbon atom?
A)5.3 1012
B)5.3 1010
C)5.3 108
D)5.3 106
E)5.3 103
A)5.3 1012
B)5.3 1010
C)5.3 108
D)5.3 106
E)5.3 103
5.3 108
4
The diameter of a carbon atom is approximately 140 pm,whereas the diameter of a pencil lead is approximately 0.7 mm.How many carbon nuclei would be required to span 0.70 mm? The radius of the nucleus is approximately 10,000 times smaller than the radius of an atom.
A)5 109 nuclei
B)5 1010 nuclei
C)5 1012 nuclei
D)5 1013 nuclei
E)5 1015 nuclei
A)5 109 nuclei
B)5 1010 nuclei
C)5 1012 nuclei
D)5 1013 nuclei
E)5 1015 nuclei

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
5
Who discovered neutrons?
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements is true regarding Thomson's cathode ray experiment and the discovery of electrons?
A)Radioactivity was also discovered.
B)Magnetic fields deflected cathode rays but electric fields did not.
C)Cathode rays were split into two beams by magnetic fields.
D)The charge of the electron was determined.
E)The mass-to-charge ratio of the electron was determined.
A)Radioactivity was also discovered.
B)Magnetic fields deflected cathode rays but electric fields did not.
C)Cathode rays were split into two beams by magnetic fields.
D)The charge of the electron was determined.
E)The mass-to-charge ratio of the electron was determined.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
7
What are cathode rays?
A)electrons
B)protons
C)neutrons
D)alpha particles
E)X-rays
A)electrons
B)protons
C)neutrons
D)alpha particles
E)X-rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the correct symbol for an particle?
A)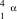
B)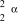
C)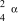
D)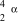
E)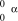
A)
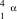
B)
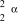
C)
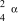
D)
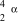
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which statement regarding Millikan's oil drop experiment is true?
A)X-rays removed electrons from fine oil droplets to produce cations.
B)The rate at which charged oil droplets fell in an adjustable magnetic field was related to the charge on an electron.
C)The rate at which charged oil droplets fell in an adjustable electric field was related to the charge on an electron.
D)The rate at which ionized N2 and O2 molecules fell between electrically charged metal plates was related to charge on an electron.
E)Electrons were found to have either a positive or negative charge depending on how they were generated.
A)X-rays removed electrons from fine oil droplets to produce cations.
B)The rate at which charged oil droplets fell in an adjustable magnetic field was related to the charge on an electron.
C)The rate at which charged oil droplets fell in an adjustable electric field was related to the charge on an electron.
D)The rate at which ionized N2 and O2 molecules fell between electrically charged metal plates was related to charge on an electron.
E)Electrons were found to have either a positive or negative charge depending on how they were generated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
10
If the nucleus of an atom had a diameter of 1 cm (roughly that of a dime),what would be the approximate diameter of the atom? The radius of the nucleus is approximately 10,000 times smaller than the radius of an atom.
A)1000 km
B)10 km
C)1000 m
D)100 m
E)1 m
A)1000 km
B)10 km
C)1000 m
D)100 m
E)1 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
11
If the nucleus of an atom has a radius of about 5 fm and a mass of about 2 10-21 g,what is its approximate density? (Volume of a sphere = 4 r3/3)
A)4 1015 g/cm3
B)4 1012 g/cm3
C)4 109 g/cm3
D)4 106 g/cm3
E)4 103 g/cm3
A)4 1015 g/cm3
B)4 1012 g/cm3
C)4 109 g/cm3
D)4 106 g/cm3
E)4 103 g/cm3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following is NOT true regarding cations and anions?
A)N3- represents a nitrogen atom that has gained three electrons.
B)An oxygen molecule can lose an electron to form O2-.
C)Ca2+ represents a calcium atom that has lost two electrons.
D)The formation of a chlorine anion can be written as Cl + e- Cl-.
E)N2 N2+ + e-describes a nitrogen molecule forming a +1 cation.
A)N3- represents a nitrogen atom that has gained three electrons.
B)An oxygen molecule can lose an electron to form O2-.
C)Ca2+ represents a calcium atom that has lost two electrons.
D)The formation of a chlorine anion can be written as Cl + e- Cl-.
E)N2 N2+ + e-describes a nitrogen molecule forming a +1 cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the correct symbol for an electron?
A)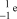
B)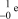
C)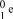
D)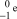
E)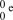
A)
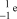
B)
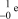
C)
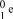
D)
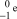
E)
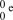

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
14
If the diameter of a carbon atom is approximately 140 pm,how many carbon atoms lined up side to side would span a pencil lead with a diameter of about 0.7 mm?
A)5 101 atoms
B)5 103 atoms
C)5 105 atoms
D)5 106 atoms
E)5 109 atoms
A)5 101 atoms
B)5 103 atoms
C)5 105 atoms
D)5 106 atoms
E)5 109 atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
15
Who was the first scientist to determine the charge of an electron?
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which subatomic particles have approximately equal masses?
A)protons and neutrons
B)protons and electrons
C)neutrons and electrons
D)protons,neutrons,and electrons
E)none of the above
A)protons and neutrons
B)protons and electrons
C)neutrons and electrons
D)protons,neutrons,and electrons
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the correct symbol for a proton?
A)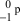
B)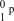
C)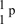
D)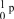
E)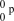
A)
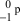
B)
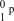
C)
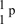
D)
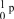
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which statement regarding the Geiger-Marsden experiment is false?
A)Beta particles were occasionally deflected by electrons in the gold atoms.
B)Alpha particles were occasionally deflected by small positively charged regions in the gold atoms.
C)The results suggested that the positive charge of an atom is localized in a small region.
D)The results suggested that most of the mass of an atom is contained in a small region.
E)The results suggested that the plum-pudding model of the atom was incorrect.
A)Beta particles were occasionally deflected by electrons in the gold atoms.
B)Alpha particles were occasionally deflected by small positively charged regions in the gold atoms.
C)The results suggested that the positive charge of an atom is localized in a small region.
D)The results suggested that most of the mass of an atom is contained in a small region.
E)The results suggested that the plum-pudding model of the atom was incorrect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
19
Who discovered electrons?
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick
A)Henri Becquerel
B)Robert Millikan
C)Joseph John Thomson
D)John Dalton
E)James Chadwick

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which statement regarding Rutherford's nuclear model of the atom is false?
A)The diameter of the atom is approximately 10,000 times larger than the nucleus.
B)The nucleus is approximately 100 times smaller than the diameter of the atom.
C)The nucleus is surrounded by a diffuse cloud of electrons.
D)Electrons and protons are not mixed uniformly throughout the atom.
E)The atom is mostly empty space.
A)The diameter of the atom is approximately 10,000 times larger than the nucleus.
B)The nucleus is approximately 100 times smaller than the diameter of the atom.
C)The nucleus is surrounded by a diffuse cloud of electrons.
D)Electrons and protons are not mixed uniformly throughout the atom.
E)The atom is mostly empty space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following statements regarding the discovery of isotopes is false?
A)Positively charged ions were deflected by a combination of electric and magnetic fields.
B)Nuclides with equal charges but different masses were deflected to different degrees.
C)The amount of deflection of an ion depended on its charge.
D)An estimate of the relative abundance of the different isotopes of an element could be ascertained.
E)If nuclides had the same mass but different positive charges,the ion with the smallest charge was deflected the most.
A)Positively charged ions were deflected by a combination of electric and magnetic fields.
B)Nuclides with equal charges but different masses were deflected to different degrees.
C)The amount of deflection of an ion depended on its charge.
D)An estimate of the relative abundance of the different isotopes of an element could be ascertained.
E)If nuclides had the same mass but different positive charges,the ion with the smallest charge was deflected the most.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
22
A 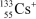 ion has __________ protons,__________ neutrons,and __________ electrons.
ion has __________ protons,__________ neutrons,and __________ electrons.
A)55,78,54
B)55,78,55
C)55,133,54
D)54,78,55
E)54,133,55
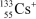 ion has __________ protons,__________ neutrons,and __________ electrons.
ion has __________ protons,__________ neutrons,and __________ electrons.A)55,78,54
B)55,78,55
C)55,133,54
D)54,78,55
E)54,133,55

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
23
The atomic model that includes isotopes differs from Dalton's view of the atom.Which of the following statements is false?
A)The identity of an atom can be determined solely by its atomic number.
B)The identity of an isotope can be determined solely by its mass number.
C)Atoms of different elements may have the same mass numbers.
D)Atoms of different elements cannot contain the same number of protons.
E)The different isotopes of an element are not always equally abundant.
A)The identity of an atom can be determined solely by its atomic number.
B)The identity of an isotope can be determined solely by its mass number.
C)Atoms of different elements may have the same mass numbers.
D)Atoms of different elements cannot contain the same number of protons.
E)The different isotopes of an element are not always equally abundant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
24
Isotopes have _____________
A)the same atomic mass.
B)the same total number of protons and neutrons.
C)the same number of neutrons but a different number of protons.
D)the same number of protons but a different number of neutrons.
E)the same number of protons but different numbers of electrons.
A)the same atomic mass.
B)the same total number of protons and neutrons.
C)the same number of neutrons but a different number of protons.
D)the same number of protons but a different number of neutrons.
E)the same number of protons but different numbers of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the correct symbol for a neutron?
A)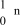
B)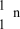
C)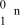
D)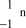
E)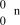
A)
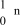
B)
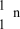
C)
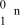
D)
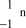
E)
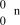

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
26
Nuclei with certain numbers of protons and neutrons (or combinations thereof)appear to be more stable than others."Magic numbers" that are consistent with known nuclides are 2,8,20,28,50,82,and 126,with 180 and 306 being hypothesized as the next in the series.Using this information,along with your knowledge of atoms and isotopes,you try to synthesize a new atom (symbol X)based on stable combinations of nucleons.Which do you think is a likely candidate?
A)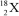
B)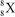
C)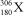
D)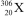
E)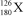
A)
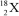
B)
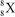
C)
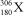
D)
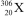
E)
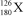

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
27
A 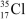 atom has __________ protons,__________ neutrons,and __________ electrons.
atom has __________ protons,__________ neutrons,and __________ electrons.
A)17,18,17
B)17,35,17
C)35,17,17
D)18,35,17
E)18,17,18
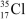 atom has __________ protons,__________ neutrons,and __________ electrons.
atom has __________ protons,__________ neutrons,and __________ electrons.A)17,18,17
B)17,35,17
C)35,17,17
D)18,35,17
E)18,17,18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
28
Identify the atom or ion: i)___________; ii)___________; and iii)___________.
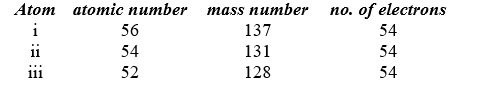
A)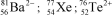
B)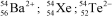
C)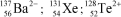
D)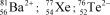
E)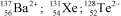
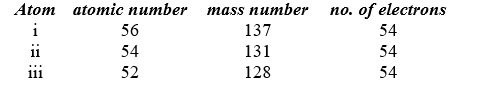
A)
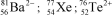
B)
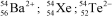
C)
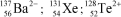
D)
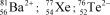
E)
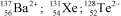

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
29
A strontium-90 atom that has a lost two electrons has __________ protons,__________ neutrons,and __________ electrons.
A)38,90,36
B)38,52,40
C)38,52,36
D)38,90,40
E)90,38,88
A)38,90,36
B)38,52,40
C)38,52,36
D)38,90,40
E)90,38,88

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which two elements would you expect to show the most similar behavior?
A)Se and Br
B)B and C
C)Li and Be
D)Sn and Bi
E)Ca and Sr
A)Se and Br
B)B and C
C)Li and Be
D)Sn and Bi
E)Ca and Sr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
31
The 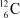 nucleus is an example of __________
nucleus is an example of __________
A)a nuclide.
B)an element.
C)a proton.
D)a neutron.
E)a nucleon.
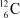 nucleus is an example of __________
nucleus is an example of __________A)a nuclide.
B)an element.
C)a proton.
D)a neutron.
E)a nucleon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
32
Cm is the symbol for __________
A)cerium.
B)chromium.
C)calcium.
D)curium.
E)cesium.
A)cerium.
B)chromium.
C)calcium.
D)curium.
E)cesium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the symbol for sulfur?
A)Si
B)Sc
C)Su
D)S
E)Sf
A)Si
B)Sc
C)Su
D)S
E)Sf

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
34
A 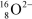 ion has __________ protons,__________ neutrons,and __________ electrons.
ion has __________ protons,__________ neutrons,and __________ electrons.
A)8,8,6
B)8,10,10
C)8,8,10
D)8,8,8
E)8,16,8
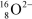 ion has __________ protons,__________ neutrons,and __________ electrons.
ion has __________ protons,__________ neutrons,and __________ electrons.A)8,8,6
B)8,10,10
C)8,8,10
D)8,8,8
E)8,16,8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
35
A phosphorus-31 atom has __________ protons,__________ neutrons,and __________ electrons.
A)31,31,31
B)15,16,16
C)15,31,15
D)15,16,15
E)16,15,15
A)31,31,31
B)15,16,16
C)15,31,15
D)15,16,15
E)16,15,15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the nuclide symbol for the atom that has an atomic number equal to the number of electrons in 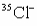 and a neutron number equal to the mass number of a sodium atom containing 11 neutrons?
and a neutron number equal to the mass number of a sodium atom containing 11 neutrons?
A)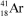
B)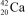
C)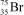
D)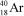
E)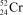
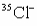 and a neutron number equal to the mass number of a sodium atom containing 11 neutrons?
and a neutron number equal to the mass number of a sodium atom containing 11 neutrons?A)
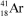
B)
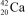
C)
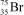
D)
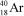
E)
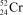

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
37
Identify the atom or ion: i)___________; ii)___________; and iii)___________.
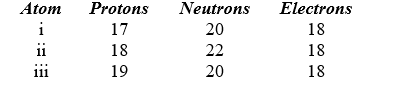
A)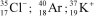
B)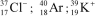
C)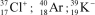
D)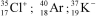
E)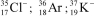
A)
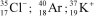
B)
C)
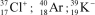
D)
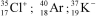
E)
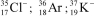

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the symbol for magnesium?
A)M
B)Mg
C)Mn
D)Mo
E)Md
A)M
B)Mg
C)Mn
D)Mo
E)Md

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the nuclide symbol for the ion that has a charge of 2+,50 neutrons more than its number of protons,and an atomic number equal to the number of electrons in a zirconium atom that has lost 2 electrons?
A)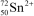
B)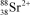
C)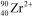
D)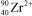
E)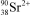
A)
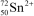
B)
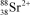
C)
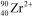
D)
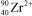
E)
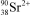

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
40
Protons and neutrons are examples of __________
A)nuclei.
B)nuclides.
C)nucleons.
D)isotopes.
E)charged particles.
A)nuclei.
B)nuclides.
C)nucleons.
D)isotopes.
E)charged particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the charge on the manganese ion in MnS2?
A)"+4"
B)"+2"
C)"+1"
D)"-1"
E)"-2"
A)"+4"
B)"+2"
C)"+1"
D)"-1"
E)"-2"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the correct formula for the compound formed between sodium and iodine based on their positions in the periodic table?
A)Na2I
B)NaI2
C)NaI
D)Na2I2
E)Na3I
A)Na2I
B)NaI2
C)NaI
D)Na2I2
E)Na3I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
43
Zinc oxide,a combination of zinc and oxygen,is found in skin ointments.What formula best describes this compound?
A)ZnO
B)Zn2O
C)ZnO2
D)Zn2O2
E)Zn2O3
A)ZnO
B)Zn2O
C)ZnO2
D)Zn2O2
E)Zn2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which element forms ionic compounds with the formula XBr2?
A)sodium
B)aluminum
C)lithium
D)calcium
E)carbon
A)sodium
B)aluminum
C)lithium
D)calcium
E)carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
45
Elements in group 17 (VIIA)are called __________
A)alkali metals.
B)pnictogens.
C)alkaline earth metal.
D)halogens.
E)chalcogens.
A)alkali metals.
B)pnictogens.
C)alkaline earth metal.
D)halogens.
E)chalcogens.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the formula for the ionic compound formed when calcium and bromine combine?
A)CBr
B)CaBr2
C)Ca2Br
D)CaBrO
E)CaB2
A)CBr
B)CaBr2
C)Ca2Br
D)CaBrO
E)CaB2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the charge on the thallium ion in Tl2O3?
A)"+1"
B)"+2"
C)"+3"
D)"-3"
E)"-1"
A)"+1"
B)"+2"
C)"+3"
D)"-3"
E)"-1"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which element forms an ionic compound with nitrogen that has the formula XN?
A)tin
B)aluminum
C)lithium
D)calcium
E)potassium
A)tin
B)aluminum
C)lithium
D)calcium
E)potassium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
49
The sixth period of the periodic table contains __________ elements.
A)18
B)32
C)24
D)16
E)8
A)18
B)32
C)24
D)16
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
50
You synthesize a superheavy atom that fits into the periodic table below radium.If it were to form an ion,what ionic charge would you predict?
A)"+2"
B)"+1"
C)"-1"
D)"-2"
E)"0 (unlikely to form an ion)"
A)"+2"
B)"+1"
C)"-1"
D)"-2"
E)"0 (unlikely to form an ion)"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
51
What is the charge on the iron ion in FeCl3?
A)"-3"
B)"+3"
C)"-1"
D)"+1"
E)"0"
A)"-3"
B)"+3"
C)"-1"
D)"+1"
E)"0"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following is NOT a common ion?
A)Rb+
B)S2-
C)Al3+
D)I-
E)Ba+
A)Rb+
B)S2-
C)Al3+
D)I-
E)Ba+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
53
Active metals often react with oxygen in air to form a protective surface film that prevents further reaction.Which one of the following formulas for a metal-oxygen combination is NOT correct?
A)Al2O3
B)Fe2O3
C)Na2O
D)MgO2
E)MnO
A)Al2O3
B)Fe2O3
C)Na2O
D)MgO2
E)MnO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
54
Elements 21 through 30 are known as __________
A)alkaline earths.
B)chalcogens.
C)halides.
D)transition metals.
E)rare earths.
A)alkaline earths.
B)chalcogens.
C)halides.
D)transition metals.
E)rare earths.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
55
Elements in group 18 (VIIIA)are called __________
A)alkali metals.
B)noble gases.
C)alkaline earth metals.
D)halogens.
E)chalcogens.
A)alkali metals.
B)noble gases.
C)alkaline earth metals.
D)halogens.
E)chalcogens.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following is an alkali metal?
A)K
B)Mg
C)Al
D)Cu
E)Ca
A)K
B)Mg
C)Al
D)Cu
E)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which element forms an ionic compound with the formula Na2X?
A)magnesium
B)carbon
C)iodine
D)phosphorus
E)sulfur
A)magnesium
B)carbon
C)iodine
D)phosphorus
E)sulfur

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
58
In what ratio will alkaline earth metals pair with halogens when they form ionic compounds?
A)3:1
B)2:1
C)1:1
D)1:2
E)1:3
A)3:1
B)2:1
C)1:1
D)1:2
E)1:3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
59
Calcium is an example of __________
A)an alkali metal.
B)a transition metal.
C)an alkaline earth metal.
D)a halogen.
E)a chalcogen.
A)an alkali metal.
B)a transition metal.
C)an alkaline earth metal.
D)a halogen.
E)a chalcogen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
60
What ion would you predict element 118 would form?
A)"+2"
B)"+1"
C)"-1"
D)"-2"
E)"0 (unlikely to form an ion)"
A)"+2"
B)"+1"
C)"-1"
D)"-2"
E)"0 (unlikely to form an ion)"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
61
Silicon is best described as a __________
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
62
Rubidium has two naturally occurring isotopes,85Rb (84.912 amu)and 87Rb (86.909 amu).Rubidium-85 is the more abundant isotope (72.17%).Calculate the average atomic mass of Rb.
A)86.91 amu
B)85.47 amu
C)85.91 amu
D)86.35 amu
E)86.00 amu
A)86.91 amu
B)85.47 amu
C)85.91 amu
D)86.35 amu
E)86.00 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
63
The average atomic mass of silver is 107.868 amu.Silver has two naturally occurring isotopes, 107Ag (106.905 amu,51.839%)and 109Ag.What is the isotopic mass of 109Ag?
A)109.11 amu
B)108.89 amu
C)108.52 amu
D)108.91 amu
E)108.48 amu
A)109.11 amu
B)108.89 amu
C)108.52 amu
D)108.91 amu
E)108.48 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
64
Oxygen is best described as a __________
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which characteristic would you expect indium NOT to exhibit?
A)shiny luster
B)electrically insulating
C)malleable
D)a +3 ionic charge
E)solid at room temperature
A)shiny luster
B)electrically insulating
C)malleable
D)a +3 ionic charge
E)solid at room temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
66
For each of the elements below,there are only two naturally occurring isotopes.Using the atomic masses on the periodic table,identify the pair in which the lighter isotope is the more abundant one.
A)"6Li and 7Li"
B)"79Br and 81Br"
C)"10B and 11B"
D)"121Sb and 123Sb"
E)"50V and 51V"
A)"6Li and 7Li"
B)"79Br and 81Br"
C)"10B and 11B"
D)"121Sb and 123Sb"
E)"50V and 51V"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
67
The average atomic mass of lithium is 6.941 amu.Lithium has two naturally occurring isotopes,6Li (7.52%)and 7Li (92.48%).The mass of 6Li is 6.0151 amu.What is the isotopic mass of 7Li?
A)7.016 amu
B)0.926 amu
C)6.001 amu
D)7.000 amu
E)6.941 amu
A)7.016 amu
B)0.926 amu
C)6.001 amu
D)7.000 amu
E)6.941 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
68
One isotope makes up 97% of all calcium atoms.Which one?
A)"40Ca"
B)"42Ca"
C)"43Ca"
D)"44Ca"
E)"48Ca"
A)"40Ca"
B)"42Ca"
C)"43Ca"
D)"44Ca"
E)"48Ca"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
69
For each of the elements below,there are only two naturally occurring isotopes.Using the atomic masses on the periodic table,identify the pair in which the heavier isotope is the more abundant one.
A)"63Cu and 65Cu"
B)"85Rb and 87Rb"
C)"10B and 11B"
D)"79Br and 81Br"
E)"14N and 15N"
A)"63Cu and 65Cu"
B)"85Rb and 87Rb"
C)"10B and 11B"
D)"79Br and 81Br"
E)"14N and 15N"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
70
Sodium is best described as a __________
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.
A)metalloid.
B)metal.
C)transition metal.
D)noble gas.
E)nonmetal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which halogen is radioactive?
A)astatine
B)polonium
C)iodine
D)tellurium
E)bismuth
A)astatine
B)polonium
C)iodine
D)tellurium
E)bismuth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the name of the halogen in period five?
A)selenium
B)tellurium
C)bromine
D)iodine
E)antimony
A)selenium
B)tellurium
C)bromine
D)iodine
E)antimony

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
73
Identify the element based on the following values for its three isotopes: 38.9637 amu (93.08%), 39.9640 amu (0.012%),and 40.9618 amu (6.91%).
A)K
B)Cl
C)S
D)Ar
E)Ca
A)K
B)Cl
C)S
D)Ar
E)Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
74
You create a superheavy atom with an atomic number of 120.To which category does it belong?
A)halogens
B)actinides
C)transition metals
D)alkali metals
E)alkaline earth metals
A)halogens
B)actinides
C)transition metals
D)alkali metals
E)alkaline earth metals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the name of the metalloid in period four that is in the same family as nitrogen?
A)bismuth
B)antimony
C)arsenic
D)carbon
E)selenium
A)bismuth
B)antimony
C)arsenic
D)carbon
E)selenium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
76
Gallium has two naturally occurring isotopes with the following masses and natural abundances.Calculate the average atomic mass of Ga. 69Ga 68.9256 amu 60.108%
71Ga 70.9247 amu 39.892%
A)69.925 amu
B)70.127 amu
C)70.000 amu
D)69.824 amu
E)69.723 amu
71Ga 70.9247 amu 39.892%
A)69.925 amu
B)70.127 amu
C)70.000 amu
D)69.824 amu
E)69.723 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
77
You create a superheavy atom with an atomic number of 120.What is probably true about that element?
A)It is probably a gas.
B)It is probably a metalloid.
C)It is probably nonmetallic.
D)It is probably metallic.
E)It probably forms a stable +1 cation.
A)It is probably a gas.
B)It is probably a metalloid.
C)It is probably nonmetallic.
D)It is probably metallic.
E)It probably forms a stable +1 cation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
78
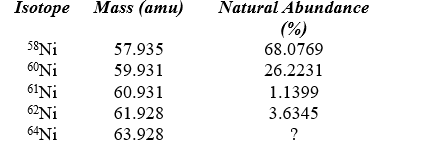
The average atomic mass of nickel is 58.693 amu.Given the data in the following table,what is the natural abundance of nickel-64?
A)92.56%
B)9.256%
C)7.440%
D)0.9256%
E)0.7440%

A)92.56%
B)9.256%
C)7.440%
D)0.9256%
E)0.7440%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
79
Cobalt is best described as a __________
A)metalloid.
B)transition metal.
C)chalcogen.
D)noble gas.
E)nonmetal.
A)metalloid.
B)transition metal.
C)chalcogen.
D)noble gas.
E)nonmetal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck
80
Identify the element based on the following values for its five isotopes: 179.947 amu (0.12%), 181.948 amu (26.50%),182.950 amu (14.31%),183.951 amu (30.64%),and 185.954 amu (28.43%).
A)Ir
B)Os
C)Re
D)Ta
E)W
A)Ir
B)Os
C)Re
D)Ta
E)W

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 157 في هذه المجموعة.
فتح الحزمة
k this deck








