Deck 21: Nuclear Chemistry-The Risks and Benefits
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/198
العب
ملء الشاشة (f)
Deck 21: Nuclear Chemistry-The Risks and Benefits
1
What other particle is formed during the fusion of two protons to form deuterium?
A)"proton"
B)"neutron"
C)"electron"
D)"positron"
E)" ray"
A)"proton"
B)"neutron"
C)"electron"
D)"positron"
E)" ray"
"positron"
2
Nuclear fusion produces energy because ________
A)neutrons are produced.
B)the total mass of the products is less than that of the reactants.
C)the total mass of the products is more than that of the reactants.
D)it is a very powerful chemical reaction.
E)photons are produced.
A)neutrons are produced.
B)the total mass of the products is less than that of the reactants.
C)the total mass of the products is more than that of the reactants.
D)it is a very powerful chemical reaction.
E)photons are produced.
the total mass of the products is less than that of the reactants.
3
What is the correct symbol for a positron?
A)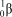
B)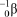
C)
D)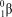
E)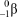
A)
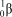
B)
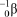
C)

D)
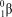
E)
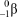
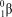
4
Why are higher temperatures necessary for fusion of helium nuclei than for hydrogen nuclei?
A)Helium is chemically inert.
B)Helium nuclei are heavier than hydrogen nuclei.
C)The boiling point of helium is higher than that of hydrogen.
D)Because E = mc2 and helium nuclei are heavier than hydrogen nuclei.
E)The positive charges on helium nuclei are greater than those on the hydrogen nuclei.
A)Helium is chemically inert.
B)Helium nuclei are heavier than hydrogen nuclei.
C)The boiling point of helium is higher than that of hydrogen.
D)Because E = mc2 and helium nuclei are heavier than hydrogen nuclei.
E)The positive charges on helium nuclei are greater than those on the hydrogen nuclei.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the correct symbol for a particle?
A)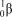
B)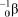
C)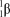
D)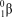
E)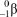
A)
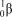
B)
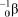
C)
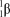
D)
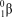
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
6
Hydrogen fusion always produces ________
A)"an increase of mass number."
B)" particles."
C)"a new element."
D)"a change in the atomic number."
E)"positrons."
A)"an increase of mass number."
B)" particles."
C)"a new element."
D)"a change in the atomic number."
E)"positrons."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements regarding Einstein's equation relating mass and energy is correct?
A)Ordinary chemical change occurs too slowly for the conversion of mass to energy to be observed.
B)The amount of energy available from a nuclear reaction decreases as the change in mass increases.
C)Small changes in mass result in very large changes in energy because of the speed of light squared dependence.
D)The mass of the universe must remain constant even though energy is released during nuclear change.
E)The energy of the universe is decreasing because the mass of the universe is increasing.
A)Ordinary chemical change occurs too slowly for the conversion of mass to energy to be observed.
B)The amount of energy available from a nuclear reaction decreases as the change in mass increases.
C)Small changes in mass result in very large changes in energy because of the speed of light squared dependence.
D)The mass of the universe must remain constant even though energy is released during nuclear change.
E)The energy of the universe is decreasing because the mass of the universe is increasing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
8
What particle is formed during the fusion of a proton and a neutron?
A)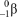
B)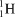
C)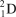
D)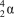
E)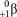
A)
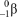
B)
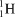
C)
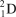
D)
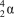
E)
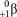

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
9
________ are elementary particles that act as carriers for the force between quarks.
A)Muons
B)Mesons
C)Leptons
D)Higgs bosons
E)Gluons
A)Muons
B)Mesons
C)Leptons
D)Higgs bosons
E)Gluons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following reactions is NOT correct?
A)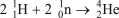
B)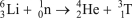
C)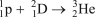
D)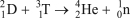
E)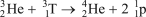
A)
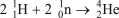
B)
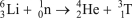
C)
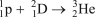
D)
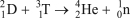
E)
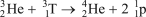

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
11
High-energy radiation produced during nuclear change consists of ________
A)"cosmic rays."
B)" rays."
C)"gluons."
D)"electrons."
E)"positrons."
A)"cosmic rays."
B)" rays."
C)"gluons."
D)"electrons."
E)"positrons."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
12
What repulsive forces must be overcome for any element other than hydrogen to exist?
A)The repulsion between neutrons and other neutrons.
B)The repulsion between protons and other protons.
C)The repulsion between protons and neutrons.
D)The repulsion between positrons and electrons.
E)The repulsion between neutrons and electrons.
A)The repulsion between neutrons and other neutrons.
B)The repulsion between protons and other protons.
C)The repulsion between protons and neutrons.
D)The repulsion between positrons and electrons.
E)The repulsion between neutrons and electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which statement is NOT correct? During primordial nucleosynthesis,________
A)neutrons and protons fused together to form deuterons.
B)deuterons fused together to form alpha particles.
C)more stable nuclides were formed from less stable nuclides.
D)gamma rays were produced.
E)colliding pairs of electrons annihilated each other.
A)neutrons and protons fused together to form deuterons.
B)deuterons fused together to form alpha particles.
C)more stable nuclides were formed from less stable nuclides.
D)gamma rays were produced.
E)colliding pairs of electrons annihilated each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
14
As the charges of identical small nuclei increase,the energy required to initiate nuclear fusion of them ________
A)decreases.
B)increases.
C)stays the same.
D)is proportional to their mass.
E)is inversely proportional to their mass.
A)decreases.
B)increases.
C)stays the same.
D)is proportional to their mass.
E)is inversely proportional to their mass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
15
The nucleus is held together by ________
A)the strong nuclear force.
B)the electromagnetic force.
C)the electroweak force.
D)Higgs bosons.
E)quarks.
A)the strong nuclear force.
B)the electromagnetic force.
C)the electroweak force.
D)Higgs bosons.
E)quarks.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
16
When a positron and an electron collide,they ________
A)form a neutron.
B)strongly repel one another and recoil.
C)annihilate each other and produce rays.
D)form a proton.
E)form a neutron and emit rays.
A)form a neutron.
B)strongly repel one another and recoil.
C)annihilate each other and produce rays.
D)form a proton.
E)form a neutron and emit rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following statements regarding ordinary chemical reactions and nuclear reactions is correct?
A)The identity of an atom may change during a nuclear reaction.
B)The identity of an atom may change during an ordinary chemical reaction.
C)There can never be a change in the total mass measured before and after a chemical reaction or a nuclear reaction.
D)The energy change associated with a chemical reaction is always much greater than that of a nuclear reaction.
E)The free energy of the universe always increases during nuclear reactions.
A)The identity of an atom may change during a nuclear reaction.
B)The identity of an atom may change during an ordinary chemical reaction.
C)There can never be a change in the total mass measured before and after a chemical reaction or a nuclear reaction.
D)The energy change associated with a chemical reaction is always much greater than that of a nuclear reaction.
E)The free energy of the universe always increases during nuclear reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
18
Extremely high temperatures are required to initiate nuclear fusion,but then energy is released by nuclear fusion.This is analogous to ________
A)boiling a liquid by heating it.
B)melting a solid by heating it.
C)lighting a match to start a fire.
D)two magnets attracting each other.
E)climbing up a hill and then skiing down.
A)boiling a liquid by heating it.
B)melting a solid by heating it.
C)lighting a match to start a fire.
D)two magnets attracting each other.
E)climbing up a hill and then skiing down.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following is thought to give particles of matter their observed masses?
A)muons
B)mesons
C)leptons
D)Higgs bosons
E)gluons
A)muons
B)mesons
C)leptons
D)Higgs bosons
E)gluons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which statement is NOT correct? In hydrogen fusion in our sun,________
A)a neutron and a proton fuse together to form a deuteron.
B)two protons fuse together to form a deuteron and a positron.
C)a deuteron and a proton fuse to produce a helium-3 nuclide.
D)two helium-3 nuclides fuse to produce a helium-4 nuclide and two protons.
E)more stable nuclides are produced from less stable nuclides.
A)a neutron and a proton fuse together to form a deuteron.
B)two protons fuse together to form a deuteron and a positron.
C)a deuteron and a proton fuse to produce a helium-3 nuclide.
D)two helium-3 nuclides fuse to produce a helium-4 nuclide and two protons.
E)more stable nuclides are produced from less stable nuclides.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
21
The most naturally abundant nuclides are those that ________
A)have an odd number of protons and an odd number of neutrons.
B)have an even number of protons and an even number of neutrons.
C)have noble gas configurations of electrons.
D)can be produced by fission reactions.
E)can be produced by fusion reactions.
A)have an odd number of protons and an odd number of neutrons.
B)have an even number of protons and an even number of neutrons.
C)have noble gas configurations of electrons.
D)can be produced by fission reactions.
E)can be produced by fusion reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
22
A barrel of oil produces about 5.9  106 kJ of energy.Fission of 235 g of uranium-235 releases 2.1
106 kJ of energy.Fission of 235 g of uranium-235 releases 2.1  1010 kJ of energy.How many barrels of oil are equivalent to 1.0 kg of uranium-235?
1010 kJ of energy.How many barrels of oil are equivalent to 1.0 kg of uranium-235?
A)3600
B)15,000
C)840,000
D)110,000
E)55,000
 106 kJ of energy.Fission of 235 g of uranium-235 releases 2.1
106 kJ of energy.Fission of 235 g of uranium-235 releases 2.1  1010 kJ of energy.How many barrels of oil are equivalent to 1.0 kg of uranium-235?
1010 kJ of energy.How many barrels of oil are equivalent to 1.0 kg of uranium-235?A)3600
B)15,000
C)840,000
D)110,000
E)55,000

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
23
When carbon burns to produce carbon dioxide,395 kJ/mol of energy is released.Approximately how much greater is the energy released per mole of deuterium in the following fusion process: 2 2H 4He? The atomic masses are 2.0141 g/mol for 2H and 4.0026 g/mol for 4He.(1 kJ = 1000 kg m2/s2)
A)102
B)104
C)106
D)108
E)1010
A)102
B)104
C)106
D)108
E)1010

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
24
The isotope "belt of stability" is an area in a plot of ________
A)nuclear binding energy vs.atomic number.
B)isotopic strength vs.isotopic weakness.
C)nuclear mass vs.mass number.
D)neutron number vs.atomic number.
E)mass number vs.atomic number.
A)nuclear binding energy vs.atomic number.
B)isotopic strength vs.isotopic weakness.
C)nuclear mass vs.mass number.
D)neutron number vs.atomic number.
E)mass number vs.atomic number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
25
What quantity of energy would be produced as one atom of plutonium-238 undergoes decay? Round your answer to two significant figures.

A)"6.0 10-7 J"
10-7 J"
B)"2.6 10-8 J"
10-8 J"
C)"7.0 10-10 J"
10-10 J"
D)"1.1 10-12 J"
10-12 J"
E)"4.9 10-12 J"
10-12 J"

A)"6.0
 10-7 J"
10-7 J"B)"2.6
 10-8 J"
10-8 J"C)"7.0
 10-10 J"
10-10 J"D)"1.1
 10-12 J"
10-12 J"E)"4.9
 10-12 J"
10-12 J"
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
26
The peak in nuclear binding energy/nucleon occurs for an isotope of ________
A)helium.
B)carbon.
C)uranium.
D)iron.
E)lead.
A)helium.
B)carbon.
C)uranium.
D)iron.
E)lead.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
27
Compare the energy released by the collisions and annihilation of (1)an electron and a positron and (2)a proton and an antiproton.
A)The energy released would be the same.
B)The energy released in (2)would be much greater than that released in (1).
C)The energy released in (1)would be much greater than that released in (2).
D)Energy is consumed in these processes,not released.
E)Energy is not released in these collisions.
A)The energy released would be the same.
B)The energy released in (2)would be much greater than that released in (1).
C)The energy released in (1)would be much greater than that released in (2).
D)Energy is consumed in these processes,not released.
E)Energy is not released in these collisions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
28
Suppose the reaction 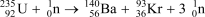 produces 1.664
produces 1.664  1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998
1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998  108 m/s; 1 kg = 6.0221415
108 m/s; 1 kg = 6.0221415  1026 amu; 1 J = 1kg . m2/s2.
1026 amu; 1 J = 1kg . m2/s2.
A)0.185 g
B)0.555 g
C)0.898 g
D)5.41 g
E)9.22 10-17 g
10-17 g
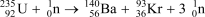 produces 1.664
produces 1.664  1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998
1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998  108 m/s; 1 kg = 6.0221415
108 m/s; 1 kg = 6.0221415  1026 amu; 1 J = 1kg . m2/s2.
1026 amu; 1 J = 1kg . m2/s2.A)0.185 g
B)0.555 g
C)0.898 g
D)5.41 g
E)9.22
 10-17 g
10-17 g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
29
When bombarded by a proton,a lithium-7 nucleus reacts to form two particles.How many kilojoules per gram of Li-7 are released during this nuclear transformation? (1 kJ = 1000 kg m2/s2)

A)1.7 109 kJ/g
109 kJ/g
B)3.9 104 kJ/g
104 kJ/g
C)5.6 10-9 kJ/g
10-9 kJ/g
D)7.9 105 kJ/g
105 kJ/g
E)2.4 108 kJ/g
108 kJ/g

A)1.7
 109 kJ/g
109 kJ/gB)3.9
 104 kJ/g
104 kJ/gC)5.6
 10-9 kJ/g
10-9 kJ/gD)7.9
 105 kJ/g
105 kJ/gE)2.4
 108 kJ/g
108 kJ/g
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
30
What quantity of energy would be produced when one mole of deuterium reacts with one mole of tritium to form helium and a neutron? (1 kJ = 1000 kg m2/s2) 
A)1.7 1012 J
1012 J
B)5.6 106 J
106 J
C)2.8 10-9 J
10-9 J
D)9.2 1013 J
1013 J
E)3.1 108 J
108 J

A)1.7
 1012 J
1012 JB)5.6
 106 J
106 JC)2.8
 10-9 J
10-9 JD)9.2
 1013 J
1013 JE)3.1
 108 J
108 J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following statements regarding the use of fusion reactions to meet Earth's energy needs is NOT correct?
A)The products of fusion reactions are not hazardous as are those of fission reactions.
B)The plasmas of high-energy particles in which fusion reactions occur can be contained by magnetic fields.
C)The amount of energy available from fusion reactions is much larger than any that generated by other process.
D)The use of lithium in "breeder" blankets is a disadvantage of the technology in development.
E)The limited supply of deuterium is a disadvantage of the technology in development.
A)The products of fusion reactions are not hazardous as are those of fission reactions.
B)The plasmas of high-energy particles in which fusion reactions occur can be contained by magnetic fields.
C)The amount of energy available from fusion reactions is much larger than any that generated by other process.
D)The use of lithium in "breeder" blankets is a disadvantage of the technology in development.
E)The limited supply of deuterium is a disadvantage of the technology in development.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
32
What quantity of energy would be produced as 1.00 g of plutonium-238 undergoes decay? Round your answer to two significant figures.

A)1.2 1010 J
1010 J
B)2.7 109
109
C)3.5 108J
108J
D)4.4 1010 J
1010 J
E)6.2 10-13 J
10-13 J

A)1.2
 1010 J
1010 JB)2.7
 109
109C)3.5
 108J
108JD)4.4
 1010 J
1010 JE)6.2
 10-13 J
10-13 J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
33
When 4 protons and 4 neutrons combine to form a beryllium-8 nucleus,energy is released.Calculate the nuclear binding energy per nucleon.Here are some helpful data: 8Be isotopic mass

A)1.20 10-6 J/nucleon
10-6 J/nucleon
B)1.08 10-9 J/nucleon
10-9 J/nucleon
C)1.13 10-12 J/nucleon
10-12 J/nucleon
D)8.73 10-12 J/nucleon
10-12 J/nucleon
E)1.08 10-15 J/nucleon
10-15 J/nucleon

A)1.20
 10-6 J/nucleon
10-6 J/nucleonB)1.08
 10-9 J/nucleon
10-9 J/nucleonC)1.13
 10-12 J/nucleon
10-12 J/nucleonD)8.73
 10-12 J/nucleon
10-12 J/nucleonE)1.08
 10-15 J/nucleon
10-15 J/nucleon
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
34
The mass of a particular isotope is given by ________
A)the mass number.
B)adding the masses of protons,neutrons,and electrons.
C)dividing the mass number by 6.02 1023.
1023.
D)measuring it.
E)dividing the molar mass of the element by 6.02 1023.
1023.
A)the mass number.
B)adding the masses of protons,neutrons,and electrons.
C)dividing the mass number by 6.02
 1023.
1023.D)measuring it.
E)dividing the molar mass of the element by 6.02
 1023.
1023.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
35
All elements with Z > 83 are ________
A)synthetic.
B)produced by nuclear fission.
C)radioactive.
D)not found in nature.
E)unreactive.
A)synthetic.
B)produced by nuclear fission.
C)radioactive.
D)not found in nature.
E)unreactive.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
36
What quantity of energy would be produced as 1.00 kg of plutonium-238 undergoes decay? Round your answer to two significant figures.

A)4.4 1013 J
1013 J
B)3.5 1011 J
1011 J
C)6.2 10-10 J
10-10 J
D)1.2 1013 J
1013 J
E)2.7 1012 J
1012 J

A)4.4
 1013 J
1013 JB)3.5
 1011 J
1011 JC)6.2
 10-10 J
10-10 JD)1.2
 1013 J
1013 JE)2.7
 1012 J
1012 J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which one of the following statements is NOT correct?
A)Nucleons are held together in a nuclide by the electromagnetic force.
B)Oxygen-15 is unstable because it has too few neutrons.
C)All nuclides with Z > 83 decay into more stable nuclides with smaller Z values.
D)As the atomic number increases,the ratio of neutrons to protons in a nuclide increases.
E)Generally the number of neutrons in a nuclide equals the number of protons,or nearly so,when the atomic number is small,i.e.,Z < 18.
A)Nucleons are held together in a nuclide by the electromagnetic force.
B)Oxygen-15 is unstable because it has too few neutrons.
C)All nuclides with Z > 83 decay into more stable nuclides with smaller Z values.
D)As the atomic number increases,the ratio of neutrons to protons in a nuclide increases.
E)Generally the number of neutrons in a nuclide equals the number of protons,or nearly so,when the atomic number is small,i.e.,Z < 18.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
38
What quantity of energy would be produced when one gram of deuterium and one gram of tritium are allowed to completely react to form helium and a neutron? (1 kJ = 1000 kg m2/s2) 
A)5.6 1011 J
1011 J
B)1.7 1012 J
1012 J
C)2.8 10-9 J
10-9 J
D)9.2 1013 J
1013 J
E)3.1 108 J
108 J

A)5.6
 1011 J
1011 JB)1.7
 1012 J
1012 JC)2.8
 10-9 J
10-9 JD)9.2
 1013 J
1013 JE)3.1
 108 J
108 J
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
39
The heaviest elements will generally have a neutron-to-proton ratio about equal to ________
A)0.5.
B)0.8.
C)1.0.
D)1.3.
E)1.5.
A)0.5.
B)0.8.
C)1.0.
D)1.3.
E)1.5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
40
Light elements with Z < 20 generally have neutron-to-proton ratios about equal to ________
A)0.5.
B)0.8.
C)1.0.
D)1.3.
E)1.5.
A)0.5.
B)0.8.
C)1.0.
D)1.3.
E)1.5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
41
For which of the following isotopes would it be unlikely to observe -decay?
A)"20F"
B)"131I"
C)"27Mg"
D)"19O"
E)"13N"
A)"20F"
B)"131I"
C)"27Mg"
D)"19O"
E)"13N"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
42
Strontium-88 is the most abundant stable isotope of strontium.Strontium-90 is a particularly hazardous radioactive isotope because,as an alkali earth metal,it will substitute for calcium in bones and teeth.Predict its decay pathway.
A)" emission"
B)" - emission"
C)"positron emission"
D)" emission"
E)"X-ray emission"
A)" emission"
B)" - emission"
C)"positron emission"
D)" emission"
E)"X-ray emission"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which one of the following statements is NOT correct?
A)Carbon-10 is unstable because it has too few neutrons.
B)All nuclides with Z > 83 decay into nuclides with smaller Z values.
C)Generally,the number of neutrons in a nuclide is equal to or less than the atomic number.
D)As the atomic number increases,the ratio of neutrons to protons in a nuclide increases.
E)It is very unusual to find a nuclide with an odd number of protons and an odd number of neutrons.
A)Carbon-10 is unstable because it has too few neutrons.
B)All nuclides with Z > 83 decay into nuclides with smaller Z values.
C)Generally,the number of neutrons in a nuclide is equal to or less than the atomic number.
D)As the atomic number increases,the ratio of neutrons to protons in a nuclide increases.
E)It is very unusual to find a nuclide with an odd number of protons and an odd number of neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
44
Radon-220 decays to polonium-216.What particle is emitted?
A)"
B)"positron"
C)"neutron"
D)" "
E)" "
A)"
B)"positron"
C)"neutron"
D)" "
E)" "

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
45
Francium-226 is a -emitter.What is the product of the radioactive decay of Fr-226?
A)astatine-222
B)radon-226
C)radium-226
D)actinium-230
E)francium-225
A)astatine-222
B)radon-226
C)radium-226
D)actinium-230
E)francium-225

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
46
Sodium-22 undergoes electron capture.Identify the resulting isotope.
A)magnesium-22
B)sodium-23
C)neon-21
D)magnesium-21
E)neon-22
A)magnesium-22
B)sodium-23
C)neon-21
D)magnesium-21
E)neon-22

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
47
Nitrogen-13 decays by positron emission to produce ________
A)carbon-13.
B)oxygen-17.
C)boron-11.
D)carbon-14.
E)boron-13.
A)carbon-13.
B)oxygen-17.
C)boron-11.
D)carbon-14.
E)boron-13.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following nuclides are most likely to be unstable because they have neutron-to-proton ratios greater than that predicted by the belt of stability?
(I)carbon-14
(II)sodium-26
(III)sulfur-26
(IV)aluminum-27
(V)phosphorus-31
A)only I
B)I and II
C)II and III
D)III,IV,and V
E)all of these
(I)carbon-14
(II)sodium-26
(III)sulfur-26
(IV)aluminum-27
(V)phosphorus-31
A)only I
B)I and II
C)II and III
D)III,IV,and V
E)all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
49
Positron emission is associated with ________
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
50
For which of the following isotopes would it be unlikely to observe + decay?
A)"120Te"
B)"96Ru"
C)"106Cd"
D)"118Sn"
E)"124Xe"
A)"120Te"
B)"96Ru"
C)"106Cd"
D)"118Sn"
E)"124Xe"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
51
Beta emission is associated with ________
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
52
The first step in the disintegration of uranium-238 produces thorium-234.What particle is emitted in this reaction?
A)" particle"
B)"neutron"
C)"proton"
D)" -particle"
E)" ray"
A)" particle"
B)"neutron"
C)"proton"
D)" -particle"
E)" ray"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
53
The heaviest stable nucleus is an isotope of ________
A)platinum.
B)gold.
C)lead.
D)bismuth.
E)xenon.
A)platinum.
B)gold.
C)lead.
D)bismuth.
E)xenon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
54
For which of the following isotopes would it be unlikely to observe electron capture?
A)"28Al"
B)"7Be"
C)"49V"
D)"73As"
E)"125I"
A)"28Al"
B)"7Be"
C)"49V"
D)"73As"
E)"125I"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
55
Cobalt-60 decays to nickel-60.What particle is emitted?
A)"proton"
B)"neutron"
C)" "
D)"positron"
E)" "
A)"proton"
B)"neutron"
C)" "
D)"positron"
E)" "

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
56
Plutonium-238 is an emitter.What is the product of the radioactive decay of Pu-238?
A)thorium-230
B)uranium-234
C)curium-242
D)californium-246
E)plutonium-234
A)thorium-230
B)uranium-234
C)curium-242
D)californium-246
E)plutonium-234

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
57
In 1913,an element was known to "emanate" from radium-226 by emission and then to decay to polonium-218,also by emission.What was the unknown element?
A)radon-222
B)lead-214
C)plutonium-238
D)bismuth-214
E)lead-218
A)radon-222
B)lead-214
C)plutonium-238
D)bismuth-214
E)lead-218

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
58
Cobalt-56 decays by emitting a positron.What is the product?
A)cobalt-55
B)cobalt-56
C)nickel-56
D)iron-56
E)iron-55
A)cobalt-55
B)cobalt-56
C)nickel-56
D)iron-56
E)iron-55

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
59
Electron-capture is associated with ________
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.
A)conversion of a neutron to a proton.
B)conversion of a proton to a neutron.
C)increase in mass number.
D)decrease in mass number.
E)emission of alpha particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
60
Cobalt-60 is a radioactive isotope of Co that is used for radiation therapy.It is originally produced from iron-58,which is first converted to Fe-59.Which particle did the Fe-58 absorb to become Fe-59?
A)" particle"
B)"neutron"
C)"proton"
D)"electron"
E)" ray"
A)" particle"
B)"neutron"
C)"proton"
D)"electron"
E)" ray"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following statements regarding nuclear decay is NOT correct?
A)Radioactive decay is a random process.
B)All nuclei of a radionuclide decay at the same rate.
C)Nuclear decay always follows first-order kinetics.
D)A large number of nuclei is generally required to determine the half-life of a radionuclide.
E)In general,the more unstable the radionuclide,the shorter the half-life.
A)Radioactive decay is a random process.
B)All nuclei of a radionuclide decay at the same rate.
C)Nuclear decay always follows first-order kinetics.
D)A large number of nuclei is generally required to determine the half-life of a radionuclide.
E)In general,the more unstable the radionuclide,the shorter the half-life.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[Nt/N0] is plotted on the y-axis with time (t)on the x-axis.N0 = number of radioactive nuclides present at time = 0 and Nt = number present at time = t.
A)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_3449_a128_b44f_6bc06ccbb5bf_TB6561_11_TB6561_11.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_3449_c839_b44f_e5a41637749d_TB6561_11_TB6561_11.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_165a_b44f_49ba2631091f_TB6561_11_TB6561_11.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_3d6b_b44f_9340f315b80e_TB6561_11_TB6561_11.jpg)
E)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_647c_b44f_717c44e0b7d4_TB6561_11_TB6561_11.jpg)
A)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_3449_a128_b44f_6bc06ccbb5bf_TB6561_11_TB6561_11.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_3449_c839_b44f_e5a41637749d_TB6561_11_TB6561_11.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_165a_b44f_49ba2631091f_TB6561_11_TB6561_11.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_3d6b_b44f_9340f315b80e_TB6561_11_TB6561_11.jpg)
E)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is longer than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_647c_b44f_717c44e0b7d4_TB6561_11_TB6561_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
63
Suppose 89.4% of a sample of radioactive sodium-24 has decayed after two days.Estimate the half-life of Na-24.
A)3.89 days
B)3.24 days
C)14.8 hours
D)12.3 hours
E)1.79 days
A)3.89 days
B)3.24 days
C)14.8 hours
D)12.3 hours
E)1.79 days

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which isotope is produced when 216Po decays by emitting an particle followed by two - particles?
A)"210Po"
B)"212Po"
C)"214Po"
D)"218Po"
E)"220Po"
A)"210Po"
B)"212Po"
C)"214Po"
D)"218Po"
E)"220Po"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
65
Calfornium-249 decays to thallium-205 through a series of nuclear reactions.Only particles and - particles are emitted.How many - particles are emitted?
A)11
B)9
C)7
D)3
E)5
A)11
B)9
C)7
D)3
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
66
Rhenium-185 is a stable isotope.Rhenium-188 is a radioisotope for treatment of cancer.What type of emission is likely for this isotope?
A)" "
B)" -"
C)"positron"
D)" ray"
E)"X-ray"
A)" "
B)" -"
C)"positron"
D)" ray"
E)"X-ray"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
67
Suppose 38.0% of a sample of radioactive polonium-218 remains after 4.33 minutes.Estimate the half-life of Po-218.
A)3.30 minutes
B)1.19 minutes
C)6.20 minutes
D)1.40 minutes
E)3.10 minutes
A)3.30 minutes
B)1.19 minutes
C)6.20 minutes
D)1.40 minutes
E)3.10 minutes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
68
The half-life of chromium-51 is 27.8 days.How many half-lives have elapsed after one year?
A)2.32 t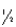
B)32.4 t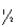
C)1.08 t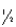
D)13.1 t
E)0.0762 t
A)2.32 t
B)32.4 t
C)1.08 t
D)13.1 t
E)0.0762 t

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
69
Cobalt-59 is a stable isotope.Cobalt-60 is used as a radioactive source approved by the FDA for irradiation of food.This process kills microbes and insects and can delay ripening.What decay pathway is likely for cobalt-60?
A)"X-ray emission"
B)" emission"
C)"positron emission"
D)" - emission"
E)" emission"
A)"X-ray emission"
B)" emission"
C)"positron emission"
D)" - emission"
E)" emission"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
70
How many and how many - particles are emitted when thorium-232 decays to form lead-208?
A)6 ,no -
B)6 ,4 -
C)12 ,8 -
D)12 ,4 -
E)8 ,6 -
A)6 ,no -
B)6 ,4 -
C)12 ,8 -
D)12 ,4 -
E)8 ,6 -

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
71
Tritium (3H)is used in glowing "EXIT" signs conveniently located where there is no electricity for lightbulbs.What decay route is likely for tritium?
A)" - emission"
B)" -emission"
C)" emission"
D)" emission"
E)"X-ray emission"
A)" - emission"
B)" -emission"
C)" emission"
D)" emission"
E)"X-ray emission"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
72
Fluorine-18,which is often used in PET scans to locate tumors,decays to form a positron and oxygen-18.After 90.0 minutes,1.00 18O is present relative to every 1.30 18F.Estimate the half-life of F-18.
A)288 minutes
B)109 minutes
C)156 minutes
D)175 minutes
E)238 minutes
A)288 minutes
B)109 minutes
C)156 minutes
D)175 minutes
E)238 minutes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
73
Predict the daughter nuclide formed when phosphorus-32 undergoes radioactive decay.
A)phosphorus-31
B)silicon-32
C)aluminum-28
D)sulfur-32
E)sodium-24
A)phosphorus-31
B)silicon-32
C)aluminum-28
D)sulfur-32
E)sodium-24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[Nt/N0] is plotted on the y-axis with time (t)on the x-axis.N0 = number of radioactive nuclides present at time = 0 and Nt = number present at time = t.
A)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_d9ad_b44f_07c90c4357d6_TB6561_11_TB6561_11.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_00be_b44f_27e0c6a58c9a_TB6561_11_TB6561_11.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_27cf_b44f_79fa63d4634d_TB6561_11_TB6561_11.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_4ee0_b44f_63c8b0a8704b_TB6561_11_TB6561_11.jpg)
E)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_75f1_b44f_0b4f9c54f565_TB6561_11_TB6561_11.jpg)
A)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344a_d9ad_b44f_07c90c4357d6_TB6561_11_TB6561_11.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_00be_b44f_27e0c6a58c9a_TB6561_11_TB6561_11.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_27cf_b44f_79fa63d4634d_TB6561_11_TB6561_11.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_4ee0_b44f_63c8b0a8704b_TB6561_11_TB6561_11.jpg)
E)
![<strong>Which graph below describes the decay of two radioactive nuclides,P and Q,when the half-life of Q is shorter than the half-life of P? In these graphs ln[N<sub>t</sub>/N<sub>0</sub>] is plotted on the y-axis with time (t)on the x-axis.N<sub>0</sub> = number of radioactive nuclides present at time = 0 and N<sub>t</sub> = number present at time = t.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_344b_75f1_b44f_0b4f9c54f565_TB6561_11_TB6561_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
75
After uranium-238 undergoes a series of eight decays and six - decays,what daughter nuclide results?
A)polonium-216
B)radon-222
C)ytterbium-182
D)osmium-182
E)lead-206
A)polonium-216
B)radon-222
C)ytterbium-182
D)osmium-182
E)lead-206

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
76
In the initial sequence of thorium-232 decay,an particle is emitted,followed by a - particle.What is the product of these two decay steps?
A)radium-228
B)actinium-228
C)thorium-228
D)francium-228
E)The correct answer differs from these possibilities.
A)radium-228
B)actinium-228
C)thorium-228
D)francium-228
E)The correct answer differs from these possibilities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
77
A half-life is ________
A)the life that a nuclear chemist leads.
B)half of the lifetime of an unstable nucleus.
C)the time for one-half of the unstable nuclei to decay.
D)constantly changing.
E)independent of the rate constant for decay.
A)the life that a nuclear chemist leads.
B)half of the lifetime of an unstable nucleus.
C)the time for one-half of the unstable nuclei to decay.
D)constantly changing.
E)independent of the rate constant for decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
78
Calfornium-249 decays to thallium-205 through a series of nuclear reactions.Only particles and - particles are emitted.How many particles are emitted?
A)22
B)18
C)15
D)11
E)9
A)22
B)18
C)15
D)11
E)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
79
Iodine-131 has a half-life of 8.1 days and is used as a tracer for the thyroid gland.If a patient drinks a sodium iodide (NaI)solution containing iodine-131,how many days will it take for the concentration of iodine-131 to drop to 5.0% of its initial concentration?
A)19 days
B)0.81 day
C)8.1 days
D)35 days
E)4.3 days
A)19 days
B)0.81 day
C)8.1 days
D)35 days
E)4.3 days

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck
80
Strontium-90 is most likely to decay by ________
A)" emission."
B)"electron capture."
C)"positron emission."
D)" emission."
E)" - emission."
A)" emission."
B)"electron capture."
C)"positron emission."
D)" emission."
E)" - emission."

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 198 في هذه المجموعة.
فتح الحزمة
k this deck








