Deck 2: Atoms, molecules, and Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/91
العب
ملء الشاشة (f)
Deck 2: Atoms, molecules, and Ions
1
Consider the following two compounds: H2O 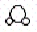 and H2O2
and H2O2 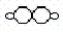 .According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 is
.According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 is
A)1:1
B)2:1
C)1:2
D)2:2
E)4:1
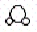 and H2O2
and H2O2 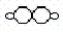 .According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 is
.According to the law of multiple proportions,the ratio of hydrogen atoms per gram of oxygen in H2O to hydrogen atoms per gram of oxygen in H2O2 isA)1:1
B)2:1
C)1:2
D)2:2
E)4:1
2:1
2
If the Thomson model of the atom had been correct,Rutherford would have observed:
A)Alpha particles going through the foil with little or no deflection.
B)Alpha particles greatly deflected by the metal foil.
C)Alpha particles bouncing off the foil.
D)Positive particles formed in the foil.
E)None of the above observations is consistent with the Thomson model of the atom.
A)Alpha particles going through the foil with little or no deflection.
B)Alpha particles greatly deflected by the metal foil.
C)Alpha particles bouncing off the foil.
D)Positive particles formed in the foil.
E)None of the above observations is consistent with the Thomson model of the atom.
Alpha particles going through the foil with little or no deflection.
3
Many classic experiments have given us indirect evidence of the nature of the atom.Which of the experiments listed below did not give the results described?
A)The Rutherford experiment proved the Thomson "plum-pudding" model of the atom to be essentially correct.
B)The Rutherford experiment was useful in determining the nuclear charge on the atom.
C)Millikan's oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.
D)The electric discharge tube proved that electrons have a negative charge.
E)All of the above experiments gave the results described.
A)The Rutherford experiment proved the Thomson "plum-pudding" model of the atom to be essentially correct.
B)The Rutherford experiment was useful in determining the nuclear charge on the atom.
C)Millikan's oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.
D)The electric discharge tube proved that electrons have a negative charge.
E)All of the above experiments gave the results described.
The Rutherford experiment proved the Thomson "plum-pudding" model of the atom to be essentially correct.
4
The Greeks proposed that matter consisted of four fundamental substances:
A)fire,earth,water,air
B)fire,metal,water,air
C)earth,metal,water,air
D)atoms,fire,water,air
E)atoms,metal,fire,air
A)fire,earth,water,air
B)fire,metal,water,air
C)earth,metal,water,air
D)atoms,fire,water,air
E)atoms,metal,fire,air

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
5
The scientist who discovered the law of conservation of mass and is also called the father of modern chemistry is
A)Proust
B)Boyle
C)Priestly
D)Bauer
E)Lavoisier
A)Proust
B)Boyle
C)Priestly
D)Bauer
E)Lavoisier

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
6
Rutherford's experiment was important because it showed that:
A)Radioactive elements give off alpha particles.
B)Gold foil can be made to be only a few atoms thick.
C)A zinc sulfide screen scintillates when struck by a charged particle.
D)The mass of the atom is uniformly distributed throughout the atom.
E)An atom is mostly empty space.
A)Radioactive elements give off alpha particles.
B)Gold foil can be made to be only a few atoms thick.
C)A zinc sulfide screen scintillates when struck by a charged particle.
D)The mass of the atom is uniformly distributed throughout the atom.
E)An atom is mostly empty space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which statement is not correct?
A)The mass of an alpha particle is 7300 times that of the electron.
B)An alpha particle has a 2+ charge.
C)Three types of radioactive emission are gamma rays,beta rays,and alpha particles.
D)A gamma ray is high-energy light.
E)There are only three types of radioactivity known to scientists today.
A)The mass of an alpha particle is 7300 times that of the electron.
B)An alpha particle has a 2+ charge.
C)Three types of radioactive emission are gamma rays,beta rays,and alpha particles.
D)A gamma ray is high-energy light.
E)There are only three types of radioactivity known to scientists today.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
8
The first people to attempt to explain why chemical changes occur were
A)alchemists
B)metallurgists
C)physicians
D)physicists
E)the Greeks
A)alchemists
B)metallurgists
C)physicians
D)physicists
E)the Greeks

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following statements about atomic structure is false?
A)An atom is mostly empty space.
B)Almost all of the mass of the atom is concentrated in the nucleus.
C)The protons and neutrons in the nucleus are very tightly packed.
D)The number of protons and neutrons is always the same in the neutral atom.
E)All of the above statements (A-D)are true.
A)An atom is mostly empty space.
B)Almost all of the mass of the atom is concentrated in the nucleus.
C)The protons and neutrons in the nucleus are very tightly packed.
D)The number of protons and neutrons is always the same in the neutral atom.
E)All of the above statements (A-D)are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
10
Alpha particles beamed at thin metal foil may
A)pass directly through without changing direction
B)be slightly diverted by attraction to electrons
C)be reflected by direct contact with nuclei
D)A and C
E)A,B,and C
A)pass directly through without changing direction
B)be slightly diverted by attraction to electrons
C)be reflected by direct contact with nuclei
D)A and C
E)A,B,and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
11
According to the law of multiple proportions:
A)If the same two elements form two different compounds,they do so in the same ratio.
B)It is not possible for the same two elements to form more than one compound.
C)The ratio of the masses of the elements in a compound is always the same.
D)The total mass after a chemical change is the same as before the change.
E)None of these.
A)If the same two elements form two different compounds,they do so in the same ratio.
B)It is not possible for the same two elements to form more than one compound.
C)The ratio of the masses of the elements in a compound is always the same.
D)The total mass after a chemical change is the same as before the change.
E)None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following pairs can be used to illustrate the law of multiple proportions?
A)SO and SO2
B)CO and CaCO3
C)H2O and C12H22O11
D)H2SO4 and H2S
E)KCl and KClO2
A)SO and SO2
B)CO and CaCO3
C)H2O and C12H22O11
D)H2SO4 and H2S
E)KCl and KClO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
13
The first scientist to show that atoms emit any negative particles was
A)J.J.Thomson
B)Lord Kelvin
C)Ernest Rutherford
D)William Thomson
E)John Dalton
A)J.J.Thomson
B)Lord Kelvin
C)Ernest Rutherford
D)William Thomson
E)John Dalton

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
14
The chemist credited for inventing a set of symbols for writing elements and a system for writing the formulas of compounds (and for discovering selenium,silicon,and thorium)is
A)Boyle
B)Lavoisier
C)Priestly
D)Berzelius
E)Dalton
A)Boyle
B)Lavoisier
C)Priestly
D)Berzelius
E)Dalton

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
15
How many of the following postulates of Dalton's atomic theory are still scientifically accepted?
I.All atoms of the same element are identical.
II.Compounds are combinations of different atoms.
III.A chemical reaction changes the way atoms are grouped together.
IV.Atoms are indestructible.
A)0
B)1
C)2
D)3
E)4
I.All atoms of the same element are identical.
II.Compounds are combinations of different atoms.
III.A chemical reaction changes the way atoms are grouped together.
IV.Atoms are indestructible.
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
16
The scientist whose alpha-particle scattering experiment led him to conclude that the nucleus of an atom contains a dense center of positive charge is
A)J.J.Thomson
B)Lord Kelvin
C)Ernest Rutherford
D)William Thomson
E)John Dalton
A)J.J.Thomson
B)Lord Kelvin
C)Ernest Rutherford
D)William Thomson
E)John Dalton

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following pairs of compounds can be used to illustrate the law of multiple proportions?
A)NH4 and NH4Cl
B)ZnO2 and ZnCl2
C)H2O and HCl
D)NO and NO2
E)CH4 and CO2
A)NH4 and NH4Cl
B)ZnO2 and ZnCl2
C)H2O and HCl
D)NO and NO2
E)CH4 and CO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following statements from Dalton's atomic theory is no longer true,according to modern atomic theory?
A)Elements are made up of tiny particles called atoms.
B)Atoms are not created or destroyed in chemical reactions.
C)All atoms of a given element are identical.
D)Atoms are indivisible in chemical reactions.
E)All of these statements are true according to modern atomic theory.
A)Elements are made up of tiny particles called atoms.
B)Atoms are not created or destroyed in chemical reactions.
C)All atoms of a given element are identical.
D)Atoms are indivisible in chemical reactions.
E)All of these statements are true according to modern atomic theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
19
The first chemist to perform truly quantitative experiments was
A)Paracelsus
B)Boyle
C)Priestly
D)Bauer
E)Lavoisier
A)Paracelsus
B)Boyle
C)Priestly
D)Bauer
E)Lavoisier

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
20
Avogadro's hypothesis states that:
A)Each atom of oxygen is 16 times more massive than an atom of hydrogen.
B)A given compound always contains exactly the same proportion of elements by mass.
C)When two elements form a series of compounds,the ratios of masses that combine with 1 gram of the first element can always be reduced to small whole numbers.
D)At the same temperature and pressure,equal volumes of different gases contain an equal number of particles.
E)Mass is neither created nor destroyed in a chemical reaction.
A)Each atom of oxygen is 16 times more massive than an atom of hydrogen.
B)A given compound always contains exactly the same proportion of elements by mass.
C)When two elements form a series of compounds,the ratios of masses that combine with 1 gram of the first element can always be reduced to small whole numbers.
D)At the same temperature and pressure,equal volumes of different gases contain an equal number of particles.
E)Mass is neither created nor destroyed in a chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
21
An isotope,X,of a particular element has an atomic number of 15 and a mass number of 31.Therefore:
A)X is an isotope of phosphorus.
B)X has 16 neutrons per atom.
C)X has an atomic mass of 30.973.
D)A and B.
E)A,B,and C.
A)X is an isotope of phosphorus.
B)X has 16 neutrons per atom.
C)X has an atomic mass of 30.973.
D)A and B.
E)A,B,and C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following are incorrectly paired?
A)Phosphorus,Pr
B)Palladium,Pd
C)Platinum,Pt
D)Lead,Pb
E)Potassium,K
A)Phosphorus,Pr
B)Palladium,Pd
C)Platinum,Pt
D)Lead,Pb
E)Potassium,K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
23
The number of electrons in an atom is the same for all neutral atoms of that element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following atomic symbols is incorrect?
A)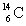
B)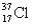
C)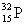
D)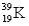
E)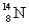
A)
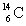
B)
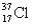
C)
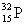
D)
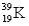
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
25
An ion is formed
A)By either adding or subtracting protons from the atom.
B)By either adding or subtracting electrons from the atom
C)By either adding or subtracting neutrons from the atom.
D)All of the above are true.
E)Two of the above are true.
A)By either adding or subtracting protons from the atom.
B)By either adding or subtracting electrons from the atom
C)By either adding or subtracting neutrons from the atom.
D)All of the above are true.
E)Two of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following are incorrectly paired?
A)Copper,Cu
B)Carbon,C
C)Cobalt,Co
D)Calcium,Ca
E)Cesium,Ce
A)Copper,Cu
B)Carbon,C
C)Cobalt,Co
D)Calcium,Ca
E)Cesium,Ce

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following are incorrectly paired?
A)K,alkali metal
B)Ba,alkaline earth metal
C)O,halogen
D)Ne,noble gas
E)Ni,transition metal
A)K,alkali metal
B)Ba,alkaline earth metal
C)O,halogen
D)Ne,noble gas
E)Ni,transition metal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
28
The numbers of protons,neutrons,and electrons in 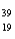 K+ are:
K+ are:
A)20 p,19 n,19 e
B)20 p,19 n,20 e
C)19 p,20 n,20 e
D)19 p,20 n,19 e
E)19 p,20 n,18 e
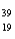 K+ are:
K+ are:A)20 p,19 n,19 e
B)20 p,19 n,20 e
C)19 p,20 n,20 e
D)19 p,20 n,19 e
E)19 p,20 n,18 e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
29
The formula of water,H2O,suggests:
A)There is twice as much mass of hydrogen as oxygen in each molecule.
B)There are two hydrogen atoms and one oxygen atom per water molecule.
C)There is twice as much mass of oxygen as hydrogen in each molecule.
D)There are two oxygen atoms and one hydrogen atom per water molecule.
E)None of these.
A)There is twice as much mass of hydrogen as oxygen in each molecule.
B)There are two hydrogen atoms and one oxygen atom per water molecule.
C)There is twice as much mass of oxygen as hydrogen in each molecule.
D)There are two oxygen atoms and one hydrogen atom per water molecule.
E)None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which among the following represent a set of isotopes? Atomic nuclei containing:
I.20 protons and 20 neutrons
II.21 protons and 19 neutrons
III.22 neutrons and 18 protons
IV.20 protons and 22 neutrons
V.21 protons and 20 neutrons
A)I,II,III
B)III,IV
C)I,V
D)I,IV and II,V
E)No isotopes are indicated.
I.20 protons and 20 neutrons
II.21 protons and 19 neutrons
III.22 neutrons and 18 protons
IV.20 protons and 22 neutrons
V.21 protons and 20 neutrons
A)I,II,III
B)III,IV
C)I,V
D)I,IV and II,V
E)No isotopes are indicated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following are incorrectly paired?
A)Sr,alkaline earth metal
B)Ta,transition metal
C)F,halogen
D)H,noble gas
E)Ru,transition metal
A)Sr,alkaline earth metal
B)Ta,transition metal
C)F,halogen
D)H,noble gas
E)Ru,transition metal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following statements are true of uranium-238? I.
Its chemical properties will be exactly like those of uranium-235.
II.
Its mass will be slightly different from that of an atom of uranium-235.
III.
It will contain a different number of protons than an atom of uranium-235.
IV.
It is more plentiful in nature than uranium-235.
A)III,IV
B)I,II,III
C)I,II,IV
D)II,III,IV
E)all of these
Its chemical properties will be exactly like those of uranium-235.
II.
Its mass will be slightly different from that of an atom of uranium-235.
III.
It will contain a different number of protons than an atom of uranium-235.
IV.
It is more plentiful in nature than uranium-235.
A)III,IV
B)I,II,III
C)I,II,IV
D)II,III,IV
E)all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
33
The number of neutrons in an atom is the same for all neutral atoms of that element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following statements is true?
A)Ions are formed by adding or removing protons or electrons.
B)Scientists believe that solids are mostly open space.
C)Heating water with a Bunsen burner results in a 2:1 mixture of hydrogen and oxygen gases.
D)At least two of the above statements (A-C)are true.
E)All of the statements (A-C)are false.
A)Ions are formed by adding or removing protons or electrons.
B)Scientists believe that solids are mostly open space.
C)Heating water with a Bunsen burner results in a 2:1 mixture of hydrogen and oxygen gases.
D)At least two of the above statements (A-C)are true.
E)All of the statements (A-C)are false.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
35
All of the following are true except:
A)Ions are formed by adding electrons to a neutral atom.
B)Ions are formed by changing the number of protons in an atom's nucleus.
C)Ions are formed by removing electrons from a neutral atom.
D)An ion has a positive or negative charge.
E)Metals tend to form positive ions.
A)Ions are formed by adding electrons to a neutral atom.
B)Ions are formed by changing the number of protons in an atom's nucleus.
C)Ions are formed by removing electrons from a neutral atom.
D)An ion has a positive or negative charge.
E)Metals tend to form positive ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
36
A species with 12 protons and 10 electrons is
A)Ne2+
B)Ti2+
C)Mg2+
D)Mg
E)Ne2-
A)Ne2+
B)Ti2+
C)Mg2+
D)Mg
E)Ne2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
37
By knowing the number of protons a neutral atom has,you should be able to determine
A)the number of neutrons in the neutral atom
B)the number of electrons in the neutral atom
C)the name of the atom
D)two of the above
E)none of the above
A)the number of neutrons in the neutral atom
B)the number of electrons in the neutral atom
C)the name of the atom
D)two of the above
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following statements is (are)true?
A)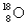 and
and 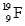 have the same number of neutrons.
have the same number of neutrons.
B)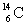 and
and 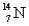 are isotopes of each other because their mass numbers are the same.
are isotopes of each other because their mass numbers are the same.
C)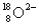 has the same number of electrons as
has the same number of electrons as  .
.
D)A and B
E)A and C
A)
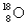 and
and 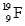 have the same number of neutrons.
have the same number of neutrons.B)
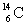 and
and 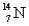 are isotopes of each other because their mass numbers are the same.
are isotopes of each other because their mass numbers are the same.C)
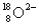 has the same number of electrons as
has the same number of electrons as  .
.D)A and B
E)A and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
39
The element rhenium (Re)exists as two stable isotopes and 18 unstable isotopes.Rhenium-185 has in its nucleus
A)75 protons,75 neutrons
B)75 protons,130 neutrons
C)130 protons,75 neutrons
D)75 protons,110 neutrons
E)not enough information
A)75 protons,75 neutrons
B)75 protons,130 neutrons
C)130 protons,75 neutrons
D)75 protons,110 neutrons
E)not enough information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
40
Bromine exists naturally as a mixture of bromine-79 and bromine-81 isotopes.An atom of bromine-79 contains
A)35 protons,44 neutrons,35 electrons
B)34 protons and 35 electrons,only
C)44 protons,44 electrons,and 35 neutrons
D)35 protons,79 neutrons,and 35 electrons
E)79 protons,79 electrons,and 35 neutrons
A)35 protons,44 neutrons,35 electrons
B)34 protons and 35 electrons,only
C)44 protons,44 electrons,and 35 neutrons
D)35 protons,79 neutrons,and 35 electrons
E)79 protons,79 electrons,and 35 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following is incorrectly named?
A)SO32-,sulfite ion
B)S2O32-,thiosulfate ion
C)PO43-,phosphate ion
D)ClO3-,chlorite ion
E)CN-,cyanide ion
A)SO32-,sulfite ion
B)S2O32-,thiosulfate ion
C)PO43-,phosphate ion
D)ClO3-,chlorite ion
E)CN-,cyanide ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following pairs is incorrect?
A)iodine trichloride,ICl3
B)phosphorus pentoxide,P2O5
C)ammonia,NH3
D)sulfur hexafluoride,SF6
E)All of the above pairs are correct.
A)iodine trichloride,ICl3
B)phosphorus pentoxide,P2O5
C)ammonia,NH3
D)sulfur hexafluoride,SF6
E)All of the above pairs are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the following are incorrectly paired?
A)Antimony,Sb
B)Silicon,Si
C)Silver,Ag
D)Argon,Ar
E)Astatine,As
A)Antimony,Sb
B)Silicon,Si
C)Silver,Ag
D)Argon,Ar
E)Astatine,As

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the subscript of barium in the formula of barium sulfate?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
45
All of the following are characteristics of nonmetals except:
A)poor conductors of electricity
B)often bond to each other by forming covalent bonds
C)tend to form negative ions in chemical reactions with metals
D)appear in the upper left-hand corner of the periodic table
E)do not have a shiny (lustrous)appearance
A)poor conductors of electricity
B)often bond to each other by forming covalent bonds
C)tend to form negative ions in chemical reactions with metals
D)appear in the upper left-hand corner of the periodic table
E)do not have a shiny (lustrous)appearance

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
46
The correct name for V3+ is
A)vanadide
B)vanadite ion
C)vanadium(III)ion
D)vanadium(V)ion
E)trivanadium ion
A)vanadide
B)vanadite ion
C)vanadium(III)ion
D)vanadium(V)ion
E)trivanadium ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
47
How many oxygen atoms are there in 4 formula units of Al2(CO3)3?
A)9
B)24
C)36
D)13
E)39
A)9
B)24
C)36
D)13
E)39

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
48
The formula for calcium bisulfate is
A)Ca(SO4)2
B)CaS2
C)Ca(HSO4)2
D)Ca2HSO4
E)Ca2S
A)Ca(SO4)2
B)CaS2
C)Ca(HSO4)2
D)Ca2HSO4
E)Ca2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
49
The correct name for FeO is
A)iron oxide
B)iron(II)oxide
C)iron(III)oxide
D)iron monoxide
E)iron(I)oxide
A)iron oxide
B)iron(II)oxide
C)iron(III)oxide
D)iron monoxide
E)iron(I)oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following is incorrectly named?
A)Pb(NO3)2,lead(II)nitrate
B)NH4ClO4,ammonium perchlorate
C)PO43-,phosphate ion
D)Mg(OH)2,magnesium hydroxide
E)NO3-,nitrite ion
A)Pb(NO3)2,lead(II)nitrate
B)NH4ClO4,ammonium perchlorate
C)PO43-,phosphate ion
D)Mg(OH)2,magnesium hydroxide
E)NO3-,nitrite ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
51
All of the following are characteristics of metals except:
A)good conductors of heat
B)malleable
C)ductile
D)often lustrous
E)tend to gain electrons in chemical reactions
A)good conductors of heat
B)malleable
C)ductile
D)often lustrous
E)tend to gain electrons in chemical reactions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following has 61 neutrons,47 protons,and 46 electrons?
A)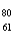 Pm
Pm
B)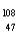 Ag+
Ag+
C)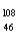 Pd-
Pd-
D)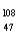 Cd+
Cd+
E)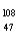 Ag
Ag
A)
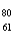 Pm
PmB)
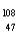 Ag+
Ag+C)
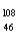 Pd-
Pd-D)
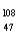 Cd+
Cd+E)
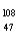 Ag
Ag
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
53
How many protons and electrons does the most stable ion for oxygen have? # protons
# electrons
A)10 p 8 e
B)8 p 6 e
C)6 p 8 e
D)8 p 8 e
E)8 p 10 e
# electrons
A)10 p 8 e
B)8 p 6 e
C)6 p 8 e
D)8 p 8 e
E)8 p 10 e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
54
The correct name for P3- is
A)phosphide ion
B)phosphorus ion
C)phosphorus(III)ion
D)phospho(III)ion
E)phosphite
A)phosphide ion
B)phosphorus ion
C)phosphorus(III)ion
D)phospho(III)ion
E)phosphite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
55
The correct name for Ca2+ is
A)calcium
B)calcium(II)ion
C)calcium ion
D)calcium(I)ion
E)monocalcium ion
A)calcium
B)calcium(II)ion
C)calcium ion
D)calcium(I)ion
E)monocalcium ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
56
The correct name for LiCl is
A)lithium monochloride
B)lithium(I)chloride
C)monolithium chloride
D)lithium chloride
E)monolithium monochloride
A)lithium monochloride
B)lithium(I)chloride
C)monolithium chloride
D)lithium chloride
E)monolithium monochloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
57
The formula for sodium dihydrogen phosphate is
A)NaH2PO4
B)Na(HPO4)2
C)NaHPO4
D)Na2HPO4
E)Na2H2PO4
A)NaH2PO4
B)Na(HPO4)2
C)NaHPO4
D)Na2HPO4
E)Na2H2PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
58
How many oxygen atoms are there in one formula unit of Ca3(PO4)2?
A)2
B)4
C)6
D)8
E)none of these
A)2
B)4
C)6
D)8
E)none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
59
You are given a compound with the formula MCl2,in which M is a metal.You are told that the metal ion has 26 electrons.What is the identity of the metal?
A)Fe
B)Al
C)Zn
D)Co
E)Ni
A)Fe
B)Al
C)Zn
D)Co
E)Ni

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following names is incorrect?
A)cobalt(II)chloride
B)magnesium oxide
C)aluminum(III)oxide
D)diphosphorus pentoxide
E)All of the above names are correct.
A)cobalt(II)chloride
B)magnesium oxide
C)aluminum(III)oxide
D)diphosphorus pentoxide
E)All of the above names are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
61
All of the following are in aqueous solution.Which is incorrectly named?
A)H2SO4,sulfuric acid
B)H2CO3,carbonic acid
C)H3PO4,phosphoric acid
D)HCN,cyanic acid
E)HCl,hydrochloric acid
A)H2SO4,sulfuric acid
B)H2CO3,carbonic acid
C)H3PO4,phosphoric acid
D)HCN,cyanic acid
E)HCl,hydrochloric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which metals form cations with varying positive charges?
A)transition metals
B)Group 1 metals
C)Group 2 metals
D)Group 3 metals
E)metalloids
A)transition metals
B)Group 1 metals
C)Group 2 metals
D)Group 3 metals
E)metalloids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
63
Name the following compounds:
Al2(SO4)3
Al2(SO4)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
64
How many atoms (total)are there in one formula unit of Ca3(PO4)2?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following pairs is incorrect?
A)NH4Br,ammonium bromide
B)K2CO3,potassium carbonate
C)BaPO4,barium phosphate
D)CuCl,copper(I)chloride
E)MnO2,manganese(IV)oxide
A)NH4Br,ammonium bromide
B)K2CO3,potassium carbonate
C)BaPO4,barium phosphate
D)CuCl,copper(I)chloride
E)MnO2,manganese(IV)oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
66
Name the following compounds:
CaSO4
CaSO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
67
Name the following compounds:
K2Cr2O7
K2Cr2O7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
68
Three samples of a solid substance composed of elements A and Z were prepared.The first contained 4.31 g A and 7.70 g Z.The second sample was 35.9% A and 64.1% Z.It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample.Show that these data illustrate the law of definite composition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
69
Explain how Dalton's atomic theory accounts for:
a)the law of conservation of mass
b)the law of definite composition
c)the law of multiple proportion
a)the law of conservation of mass
b)the law of definite composition
c)the law of multiple proportion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
70
Name the following compounds:
CCl4
CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
71
Write the chemical formulas for the following compounds or ions.
a)
nitrate ion
_________
b)
aluminum oxide
_________
c)
ammonium ion
_________
d)
perchloric acid
_________
e)
copper(II)bromide
_________
a)
nitrate ion
_________
b)
aluminum oxide
_________
c)
ammonium ion
_________
d)
perchloric acid
_________
e)
copper(II)bromide
_________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
72
Name the following compounds:
AgCl
AgCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
73
Name the following compounds:
N2O3
N2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
74
All of the following are in aqueous solution.Which is incorrectly named?
A)HC2H3O2,acetic acid
B)HBr,bromic acid
C)H2SO3,sulfurous acid
D)HNO2,nitrous acid
E)HClO3,chloric acid
A)HC2H3O2,acetic acid
B)HBr,bromic acid
C)H2SO3,sulfurous acid
D)HNO2,nitrous acid
E)HClO3,chloric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
75
Name the following compounds:
NH4NO3
NH4NO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
76
Name the following compounds:
HNO2
HNO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
77
Name the following compounds:
NaH
NaH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
78
Arsenopyrite is a mineral containing As,Fe,and S.Classify each element as metal,nonmetal,or metalloid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
79
Write the names of the following compounds:
a)
FeSO4
________________
b)
NaC2H3O2
________________
c)
KNO2
________________
d)
Ca(OH)2
________________
e)
NiCO3
________________
a)
FeSO4
________________
b)
NaC2H3O2
________________
c)
KNO2
________________
d)
Ca(OH)2
________________
e)
NiCO3
________________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck
80
Write the symbol for each of the following elements.
a)
silver
_____________
b)
calcium
_____________
c)
iodine
_____________
d)
copper
_____________
e)
phosphorus
_____________
a)
silver
_____________
b)
calcium
_____________
c)
iodine
_____________
d)
copper
_____________
e)
phosphorus
_____________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 91 في هذه المجموعة.
فتح الحزمة
k this deck








