Deck 9: Aldehydes and Ketones: Nucleophilic Addition Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/32
العب
ملء الشاشة (f)
Deck 9: Aldehydes and Ketones: Nucleophilic Addition Reactions
1
Draw structures corresponding to each of the following names.
Draw:
trans-3-isopropylcyclohexanecarbaldehyde
Draw:
trans-3-isopropylcyclohexanecarbaldehyde
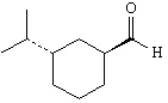
2
The substance formed in the following reaction is called: 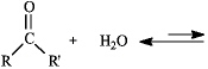
A) vicinal diol
B) geminal diol
C) acetal
D) hydrate
E) b or d
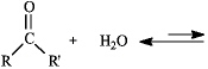
A) vicinal diol
B) geminal diol
C) acetal
D) hydrate
E) b or d
E
3
Draw structures corresponding to each of the following names.
Draw:
benzophenone
Draw:
benzophenone
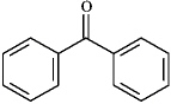
4
Instructions: Predict the products from the information given for the following question(s).
Predict: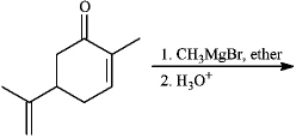
Predict:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
5
Instructions: Consider the data below to answer the following question(s). Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.  Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
A) a nucleophilic substitution
B) an electrophilic addition
C) an electrophilic substitution
D) a nucleophilic addition
 Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.A) a nucleophilic substitution
B) an electrophilic addition
C) an electrophilic substitution
D) a nucleophilic addition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
6
Provide IUPAC names for each structure below.
Name: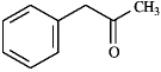
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
7
Instructions: Consider the reaction below to answer the following question. 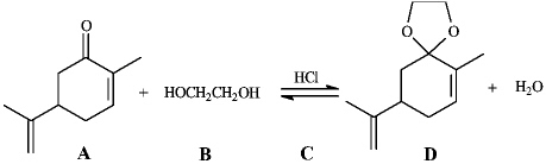 Refer to instructions. The product of this reaction is called:
Refer to instructions. The product of this reaction is called:
A) an ylide
B) an acetal
C) a gem diol
D) a hydrate
 Refer to instructions. The product of this reaction is called:
Refer to instructions. The product of this reaction is called:A) an ylide
B) an acetal
C) a gem diol
D) a hydrate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
8
Instructions: Consider the reaction below to answer the following question. 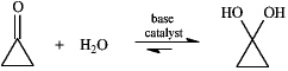 Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
 Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
9
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon, as shown below. Use this information to answer the following question(s). 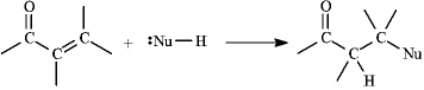 Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
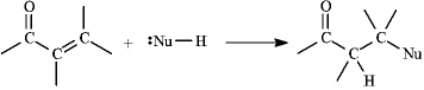 Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
10
Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by:
A) making the carbonyl group more electrophilic
B) shifting the equilibrium of the reaction
C) making the carbonyl group less electrophilic
D) converting the water to hydroxide ion, a much better nucleophile
A) making the carbonyl group more electrophilic
B) shifting the equilibrium of the reaction
C) making the carbonyl group less electrophilic
D) converting the water to hydroxide ion, a much better nucleophile

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
11
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon, as shown below. Use this information to answer the following question(s). 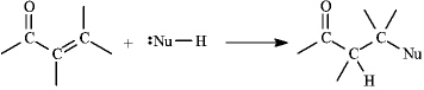 Refer to instructions. This reaction is called a(n) _____ reaction.
Refer to instructions. This reaction is called a(n) _____ reaction.
A) conjugate addition.
B) electrophilic addition.
C) direct addition
D) 1,2-addition.
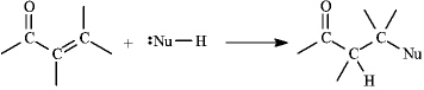 Refer to instructions. This reaction is called a(n) _____ reaction.
Refer to instructions. This reaction is called a(n) _____ reaction.A) conjugate addition.
B) electrophilic addition.
C) direct addition
D) 1,2-addition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
12
Provide IUPAC names for each structure below.
Name: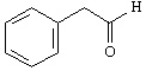
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
13
Instructions: Predict the products from the information given for the following question(s).
Predict: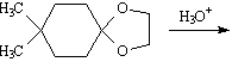
Predict:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
14
Draw structures corresponding to each of the following names.
Draw:
cyclohex-2-enone
Draw:
cyclohex-2-enone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
15
Draw structures corresponding to each of the following names.
Draw:
2,2-dimethylcyclopentane-1,3-dione
Draw:
2,2-dimethylcyclopentane-1,3-dione

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
16
Provide IUPAC names for each structure below.
Name: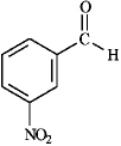
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
17
Provide IUPAC names for each structure below.
Name: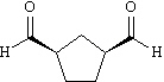
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
18
Draw structures corresponding to each of the following names.
Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)
Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
19
Instructions: Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.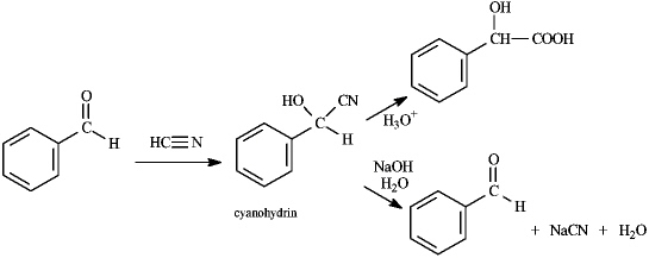 Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.
 Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
20
Instructions: Consider the reaction below to answer the following question. 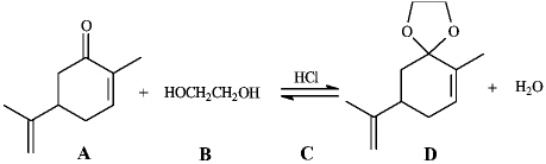 Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
 Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
21
Predict the products of the following reactions. 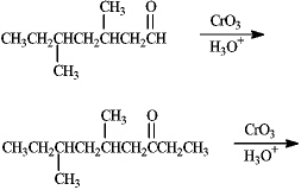


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
22
Instructions: Predict the products from the information given for the following question(s).
Predict: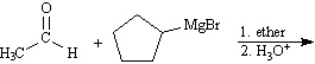
Predict:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
23
The nucleophillic addition of water to an aldehyde or ketone
A) is irreversible.
B) dependent on the structure of the carbonyl.
C) favored by neutral conditions.
D) produces a stable tetrahedral product.
E) all of these.
A) is irreversible.
B) dependent on the structure of the carbonyl.
C) favored by neutral conditions.
D) produces a stable tetrahedral product.
E) all of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the correct structure for 2-hydroxyacetophenone? 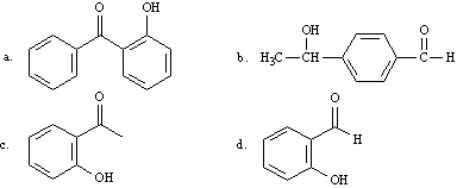


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
25
Predict the products of the following reactions. 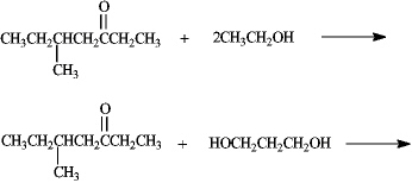


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
26
Outline the synthetic steps necessary to carry out the conversion below. You may use any organic or inorganic reagents you need. Show the structures of all intermediate compounds that would probably be isolated during the course of your synthesis, and show all necessary reagents 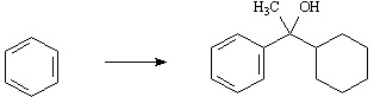


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
27
What hemiacetal would form from the internal nucleophillic addition reaction of the following compound? 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the IUPAC name of the following compound? 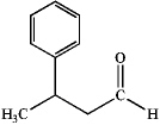
A) 3-methyl-3-phenylpropanol
B) 3-phenylbutanal
C) 3-phenyl-1-butanone
D) 3-phenylbutanoic acid

A) 3-methyl-3-phenylpropanol
B) 3-phenylbutanal
C) 3-phenyl-1-butanone
D) 3-phenylbutanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
29
Propose a synthesis of Dimestrol starting from p-methoxypropiophenone as the only source of carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
30
Instructions: Predict the products from the information given for the following question(s).
Predict: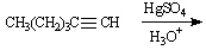
Predict:
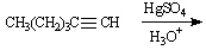

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
31
Instructions: Predict the products from the information given for the following question(s).
Predict: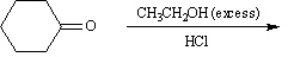
Predict:
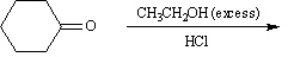

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck
32
Instructions: Predict the products from the information given for the following question(s).
Predict: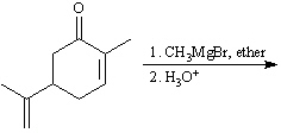
Predict:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 32 في هذه المجموعة.
فتح الحزمة
k this deck








