Deck 7: Organohalides: Nucleophilic Substitutions and Eliminations
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
Match between columns

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/48
العب
ملء الشاشة (f)
Deck 7: Organohalides: Nucleophilic Substitutions and Eliminations
1
Instructions: Provide a IUPAC name for each of the following alkyl halides.
Name:
CHI3 (iodoform)
Name:
CHI3 (iodoform)
1,1,1-triiodomethane
2
Instructions: For each question, draw a structure corresponding to the given name.
Draw:
1,2-dichloro-1,1,2,2-tetrafluoroethane (Cryofluorane)
Draw:
1,2-dichloro-1,1,2,2-tetrafluoroethane (Cryofluorane)
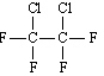
3
Instructions: Consider the pair of reactions below to answer the following question(s). 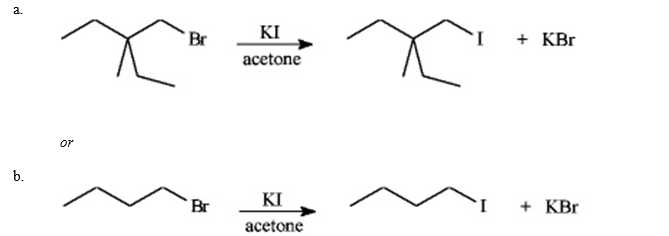 Refer to instructions. The nucleophile in these reactions is:
Refer to instructions. The nucleophile in these reactions is:
A) K+
B) alkyl group
C) Br-
D) I-
 Refer to instructions. The nucleophile in these reactions is:
Refer to instructions. The nucleophile in these reactions is:A) K+
B) alkyl group
C) Br-
D) I-
I-
4
Instructions: Consider the reaction below to answer the following question(s). 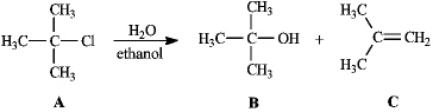 Refer to instructions. Compound B is the:
Refer to instructions. Compound B is the:
A) SN2 product
B) SN1 product
C) E2 product
D) E1 product
 Refer to instructions. Compound B is the:
Refer to instructions. Compound B is the:A) SN2 product
B) SN1 product
C) E2 product
D) E1 product

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
5
Instructions: For each question, draw a structure corresponding to the given name.
Draw:
3-iodoprop-1-ene
Draw:
3-iodoprop-1-ene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
6
Instructions: Provide a IUPAC name for each of the following alkyl halides.
Name: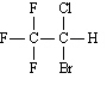 (Halothane)
(Halothane)
Name:
 (Halothane)
(Halothane)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
7
Instructions: For each question, draw a structure corresponding to the given name.
Draw:
trans-1-sec-butyl-3-chlorocyclohexane
Draw:
trans-1-sec-butyl-3-chlorocyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
8
Instructions: Provide a IUPAC name for each of the following alkyl halides.
Name: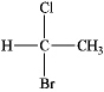
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
9
Instructions: Consider the reaction below to answer the following question(s). 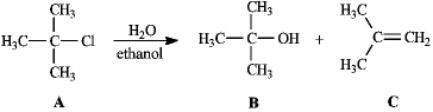 Refer to instructions. Compound C is the:
Refer to instructions. Compound C is the:
A) SN2 product
B) SN1 product
C) E2 product
D) E1 product
 Refer to instructions. Compound C is the:
Refer to instructions. Compound C is the:A) SN2 product
B) SN1 product
C) E2 product
D) E1 product

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
10
Instructions: Provide a IUPAC name for each of the following alkyl halides.
Name: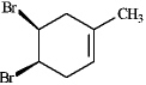
Name:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
11
Instructions: For each question, provide structures for the reactants, intermediates, or products, as indicated. Draw the structures in the boxes provided.
Draw: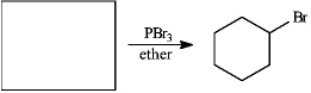
Draw:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
12
Instructions: For each question, draw a structure corresponding to the given name.
Draw:
(S)-2-bromobutane
Draw:
(S)-2-bromobutane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
13
Instructions: Provide a IUPAC name for each of the following alkyl halides.
Name:
The solvents: CCl4 and CH2Cl2
Name:
The solvents: CCl4 and CH2Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
14
Instructions: For each question, provide structures for the reactants, intermediates, or products, as indicated. Draw the structures in the boxes provided.
Draw: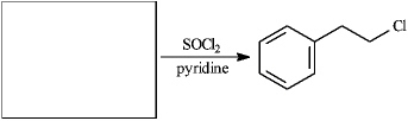
Draw:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
15
Instructions: Consider the pair of reactions below to answer the following question(s).  Refer to instructions. The mechanism for these reactions is:
Refer to instructions. The mechanism for these reactions is:
A) SN2
B) E2
C) SN1
D) E1
 Refer to instructions. The mechanism for these reactions is:
Refer to instructions. The mechanism for these reactions is:A) SN2
B) E2
C) SN1
D) E1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
16
Instructions: Consider the reaction below to answer the following question(s). 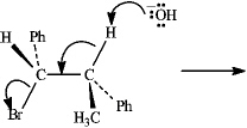 Refer to instructions. Write the product that results from the indicated electron flow in the reaction, showing any resulting stereochemistry
Refer to instructions. Write the product that results from the indicated electron flow in the reaction, showing any resulting stereochemistry
 Refer to instructions. Write the product that results from the indicated electron flow in the reaction, showing any resulting stereochemistry
Refer to instructions. Write the product that results from the indicated electron flow in the reaction, showing any resulting stereochemistry
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
17
Instructions: Circle the correct response in each set below.
Refer to instructions. The least reactive compound in an SN1 reaction.
Refer to instructions. The least reactive compound in an SN1 reaction.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
18
Instructions: Consider the pair of reactions below to answer the following question(s). 
00 Refer to instructions. The alkyl bromide starting materials in these reactions are classified as:
A) 3
B) 2
C) 1
D) 4

00 Refer to instructions. The alkyl bromide starting materials in these reactions are classified as:
A) 3
B) 2
C) 1
D) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
19
Instructions: Consider the reaction below to answer the following question. 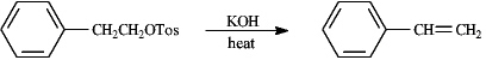 Refer to instructions. The mechanism for this reaction is:
Refer to instructions. The mechanism for this reaction is:
A) SN2
B) E2
C) SN1
D) E1
 Refer to instructions. The mechanism for this reaction is:
Refer to instructions. The mechanism for this reaction is:A) SN2
B) E2
C) SN1
D) E1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
20
Instructions: Circle the correct response in each set below.
Refer to instructions. The least reactive compound in an SN2 reaction. Explain your choice.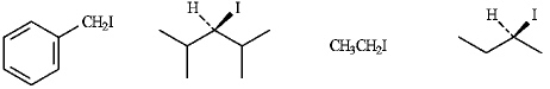
Refer to instructions. The least reactive compound in an SN2 reaction. Explain your choice.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
21
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.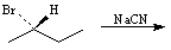
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
22
Instructions: Consider the reaction below to answer the following question(s). 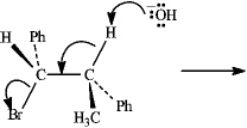 Refer to instructions. The mechanism of this reaction is:
Refer to instructions. The mechanism of this reaction is:
A) SN1
B) SN2
C) E1
D) E2
 Refer to instructions. The mechanism of this reaction is:
Refer to instructions. The mechanism of this reaction is:A) SN1
B) SN2
C) E1
D) E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
23
Consider the reaction below to answer the following questions. 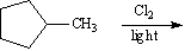 Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
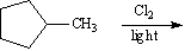 Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
24
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.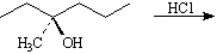
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
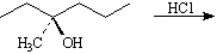

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider the following compound: 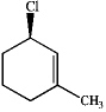




فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which mechanism is favored by the reaction of a secondary alkyl bromide with potassium t-butoxide?
A) SN1
B) SN2
C) E1
D) E1CB
E) E2
A) SN1
B) SN2
C) E1
D) E1CB
E) E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following represents the transition state of the SN2 reaction between methyl iodide and ammonia?
A)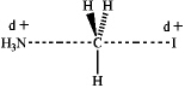
B)
C)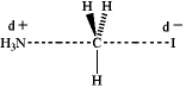
D)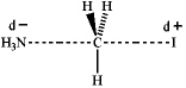
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
28
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.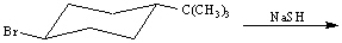
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
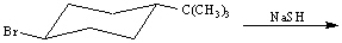

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider the reactions below to answer the following questions. a.
or
b. The mechanism for these reactions is:
a.
b.
c.
d
or
b. The mechanism for these reactions is:
a.
b.
c.
d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the IUPAC name of the following compound? 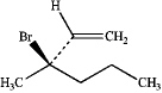
A) (R)-2-bromo-2-vinylpentane
B) (S)-2-bromo-2-vinylpentane
C) (S)-3-bromo-3-propylbut-1-ene
D) (R)-3-bromo-3-methylhex-1-ene

A) (R)-2-bromo-2-vinylpentane
B) (S)-2-bromo-2-vinylpentane
C) (S)-3-bromo-3-propylbut-1-ene
D) (R)-3-bromo-3-methylhex-1-ene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
31
Instructions: Consider the reaction below to answer the following question(s). 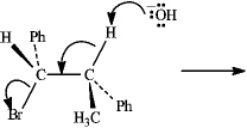 Refer to instructions. Draw a Newman projection of the reactive conformation of the starting material.
Refer to instructions. Draw a Newman projection of the reactive conformation of the starting material.
 Refer to instructions. Draw a Newman projection of the reactive conformation of the starting material.
Refer to instructions. Draw a Newman projection of the reactive conformation of the starting material.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
32
Predict the product of the following reaction: 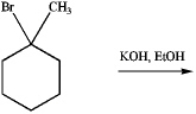


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which is most reactive in an SN1 reaction? Explain your choice.
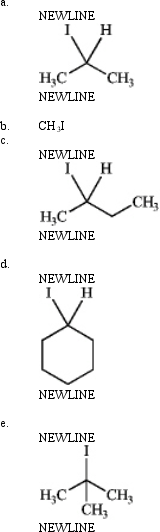


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following statements about an SN2 reaction is true?
A) the reaction occurs in two steps
B) rate = k[RX]
C) stabilization of R+ is important
D) the reaction causes racemization
E) the reaction is favored by aprotic solvents
A) the reaction occurs in two steps
B) rate = k[RX]
C) stabilization of R+ is important
D) the reaction causes racemization
E) the reaction is favored by aprotic solvents

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
35
Consider the reactions below to answer the following questions. a.
or
b. Which reaction would be predicted to be faster? Explain
or
b. Which reaction would be predicted to be faster? Explain

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following statements about an SN1 reaction is true?
A) the reaction occurs in one-step
B) there is no effect on reaction rate by nucleophile
C) primary alkyl halides react faster than secondary alkyl halides
D) the reaction proceeds with inversion of stereochemistry
E) the reaction is favored by aprotic solvents
A) the reaction occurs in one-step
B) there is no effect on reaction rate by nucleophile
C) primary alkyl halides react faster than secondary alkyl halides
D) the reaction proceeds with inversion of stereochemistry
E) the reaction is favored by aprotic solvents

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which mechanism is favored by the reaction of a tertiary alkyl chloride with ethanol?
A) SN1
B) SN2
C) E1
D) E1CB
E) E2
A) SN1
B) SN2
C) E1
D) E1CB
E) E2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
38
Consider the following reaction: 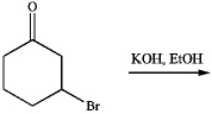 a) Draw the prochuct of the reaction. How would you cate gorize this process mechanistically?
a) Draw the prochuct of the reaction. How would you cate gorize this process mechanistically?
b) Which af the following spectral methods caud be used to follow the pargess of the reartigr??
a. NMR spectroscopy
b. Mass spectrometry
c. Infrared spectroscopy
d. UV spectroscopy
e. all of the se
f. a, b, and c onlv
 a) Draw the prochuct of the reaction. How would you cate gorize this process mechanistically?
a) Draw the prochuct of the reaction. How would you cate gorize this process mechanistically? b) Which af the following spectral methods caud be used to follow the pargess of the reartigr??
a. NMR spectroscopy
b. Mass spectrometry
c. Infrared spectroscopy
d. UV spectroscopy
e. all of the se
f. a, b, and c onlv

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
39
Consider the reactions below to answer the following questions. a.
or
b. Halving the concentration of hydroxide in these reactions:
a. causes the reaction mechanism to change
b. halves the rate of reaction
c. has no effect on the rate of reaction
d. doubles the rate of reaction
or
b. Halving the concentration of hydroxide in these reactions:
a. causes the reaction mechanism to change
b. halves the rate of reaction
c. has no effect on the rate of reaction
d. doubles the rate of reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the rate law for the E2 reaction of an alkyl halide (RX) with sodium ethoxide (NaOEt) in ethanol solvent (EtOH)?
A) rate = k[RX]
B) rate = k[RX]2
C) rate = k[RX][Na+]
D) rate = k[RX][OEt-]
E) rate = k[OEt-]
A) rate = k[RX]
B) rate = k[RX]2
C) rate = k[RX][Na+]
D) rate = k[RX][OEt-]
E) rate = k[OEt-]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
41
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.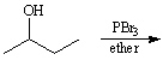
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
42
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.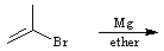
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
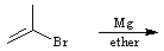

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
43
Instructions:
For each substrate below, choose which reaction type is favored. Place the letter of the reaction type in the blank above the substrate.
a.
b.
c.
d Refer to Instructions.
_____
For each substrate below, choose which reaction type is favored. Place the letter of the reaction type in the blank above the substrate.
a.
b.
c.
d Refer to Instructions.
_____

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
44
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.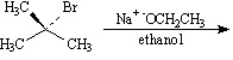
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
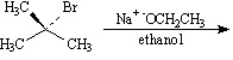

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
45
Instructions:
For each substrate below, choose which reaction type is favored. Place the letter of the reaction type in the blank above the substrate.
a.
b.
c.
d Refer to Instructions.
_____
For each substrate below, choose which reaction type is favored. Place the letter of the reaction type in the blank above the substrate.
a.
b.
c.
d Refer to Instructions.
_____

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
46
Propose a synthesis of the following compound from the given starting material and any inorganic reagents necessary. 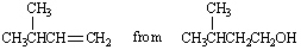
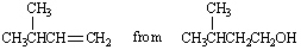

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
47
Grignard Reagents are prepared by reaction of:



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck
49
Match between columns

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 48 في هذه المجموعة.
فتح الحزمة
k this deck











