Deck 9: The Periodic Table and Some Atomic Properties
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/96
العب
ملء الشاشة (f)
Deck 9: The Periodic Table and Some Atomic Properties
1
The actinides refer to what group of elements?
A) s-block elements
B) d-block elements
C) f-block elements of atomic number 58 through 71
D) f-block elements of atomic number 90 through 103
E) p-block elements
A) s-block elements
B) d-block elements
C) f-block elements of atomic number 58 through 71
D) f-block elements of atomic number 90 through 103
E) p-block elements
f-block elements of atomic number 90 through 103
2
The inner transition elements include the actinides and the lanthanides.
True
3
The electron affinity is the energy for an atom in its standard state to gain an electron.
False
4
The electron affinity is opposite to the ionization energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
5
Why was Moseley so sure from his X-ray work that there were no other undiscovered elements besides the three that he predicted in the range of atomic numbers 13-79?
A) The rows on the periodic table were filled.
B) The columns of the periodic table were full.
C) All of the possible atomic weights were assigned.
D) All of the integer atomic numbers were assigned.
E) Mendeleev didn't predict any more.
A) The rows on the periodic table were filled.
B) The columns of the periodic table were full.
C) All of the possible atomic weights were assigned.
D) All of the integer atomic numbers were assigned.
E) Mendeleev didn't predict any more.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
6
A metallic oxide plus water forms an acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
7
Elements in the first two groups are part of the main-group elements along with which of the following groups?
A) d-block elements
B) s-block elements
C) actinides
D) lanthanides
E) p-block elements
A) d-block elements
B) s-block elements
C) actinides
D) lanthanides
E) p-block elements

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following pairs of elements are classified as metalloids?
A) aluminum and silicon
B) argon and neon
C) arsenic and germanium
D) bromine and sulfur
E) copper and silver
A) aluminum and silicon
B) argon and neon
C) arsenic and germanium
D) bromine and sulfur
E) copper and silver

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
9
Paramagnetic is not the same as magnetic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
10
The ion represented as 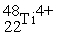 has:
has:
A) 22 electrons
B) 48 neutrons
C) 26 protons
D) 4 3d electrons
E) 0 4s electrons
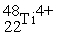 has:
has:A) 22 electrons
B) 48 neutrons
C) 26 protons
D) 4 3d electrons
E) 0 4s electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
11
Ions always adopt the electron configuration of a nobel gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
12
Moseley used X-rays to determine the atomic mass of each element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
13
The similar chemical behavior of the elements in a given group in the periodic table is best accounted for by the fact that atoms of these elements have:
A) the same number of isotopes
B) the same number of electrons
C) the same number of electrons in the outermost (valence) shell
D) similar nuclear structures
E) the same number of protons
A) the same number of isotopes
B) the same number of electrons
C) the same number of electrons in the outermost (valence) shell
D) similar nuclear structures
E) the same number of protons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
14
An atom with an even number of electrons is always diamagnetic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
15
The covalent radius, metallic radius, and the ionic radius are all equal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
16
Plutonium is among a group of elements referred to as:
A) antinides
B) alkalides
C) chalcocides
D) halides
E) lanthanides
A) antinides
B) alkalides
C) chalcocides
D) halides
E) lanthanides

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following could never be isoelectronic species?
A) anions of two different elements
B) cations of two different elements
C) an anion and a cation
D) an anion and an atom
E) atoms of two different elements
A) anions of two different elements
B) cations of two different elements
C) an anion and a cation
D) an anion and an atom
E) atoms of two different elements

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
18
Most transition elements form some monatomic ions that are not:
A) isoelectronic with any other cations
B) isoelectronic with any noble gas
C) isoelectronic with any neutral atom
D) isoelectronic with any other species
E) isoelectronic with any other transition metal ion
A) isoelectronic with any other cations
B) isoelectronic with any noble gas
C) isoelectronic with any neutral atom
D) isoelectronic with any other species
E) isoelectronic with any other transition metal ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
19
The ionization energy is the energy for a gaseous atom to lose an electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
20
The transition elements refer to what group of elements?
A) s-block elements
B) d-block elements
C) actinides
D) lanthanides
E) p-block elements
A) s-block elements
B) d-block elements
C) actinides
D) lanthanides
E) p-block elements

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following isoelectronic species has the largest radius?
A) Ne
B) F-
C) Mg2+
D) Na+
E) O2-
A) Ne
B) F-
C) Mg2+
D) Na+
E) O2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following has the largest atomic radius?
A) Rb
B) Br
C) Mo
D) I
A) Rb
B) Br
C) Mo
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following has the largest radius?
A) Se2-
B) Kr
C) Rb+
D) Br-
E) Y3+
A) Se2-
B) Kr
C) Rb+
D) Br-
E) Y3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following occurs for the representative elements going left to right across the period?
A) Electronegativity decreases.
B) Atomic size increases.
C) Forces of attraction between electron and nucleus increase because nuclear charge increases.
D) The outer electrons are held more weakly.
E) none of these
A) Electronegativity decreases.
B) Atomic size increases.
C) Forces of attraction between electron and nucleus increase because nuclear charge increases.
D) The outer electrons are held more weakly.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which comparison of atomic and/or ionic radii is correct?
A) K+ > K
B) K+ > Ca2+
C) Si > S
D) Kr > Xe
E) Cl > Cl-
A) K+ > K
B) K+ > Ca2+
C) Si > S
D) Kr > Xe
E) Cl > Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
26
The effective nuclear charge for sodium is:
A) <11, >10
B) <10, >9
C) <3, >1
D) <1, >0
E) 0
A) <11, >10
B) <10, >9
C) <3, >1
D) <1, >0
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
27
A is the sequence Na, Mg, Al, Si, and P. B is the sequence He, Ne, Ar, Kr, and Xe. Which statement below is true?
A) The members of A are decreasing in size.
B) The members of B have larger radii than those of A, respectively. That is He > Na, Ne > Mg, etc.
C) The members of A have larger radii than those of B, respectively.
D) The members of B are decreasing in size.
E) All the members of A are isoelectronic.
A) The members of A are decreasing in size.
B) The members of B have larger radii than those of A, respectively. That is He > Na, Ne > Mg, etc.
C) The members of A have larger radii than those of B, respectively.
D) The members of B are decreasing in size.
E) All the members of A are isoelectronic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
28
Choose the INCORRECT statement.
A) Within a given sublevel, each orbital is usually occupied by a single electron before any orbital has two electrons.
B) When a metallic element unites with a nonmetallic element, electrons are lost by atoms of the metal and gained by atoms of the nonmetals.
C) The rare gas atoms complete the filling of the p orbitals.
D) The ionization energy of Ca+ is greater than that for Ca++.
E) Ba2+ is smaller than Ba+.
A) Within a given sublevel, each orbital is usually occupied by a single electron before any orbital has two electrons.
B) When a metallic element unites with a nonmetallic element, electrons are lost by atoms of the metal and gained by atoms of the nonmetals.
C) The rare gas atoms complete the filling of the p orbitals.
D) The ionization energy of Ca+ is greater than that for Ca++.
E) Ba2+ is smaller than Ba+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following statements is INCORRECT?
A) The atomic radius decreases from left to right through a period of elements.
B) Cations are larger than the atoms from which they are formed.
C) The metallic character of elements decreases from left to right through a period of elements.
D) The ionization energy increases from left to right through a period of elements.
E) The atomic radius increases down the periodic table.
A) The atomic radius decreases from left to right through a period of elements.
B) Cations are larger than the atoms from which they are formed.
C) The metallic character of elements decreases from left to right through a period of elements.
D) The ionization energy increases from left to right through a period of elements.
E) The atomic radius increases down the periodic table.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the ground state electron configuration for iron(III)?
A) [Ar]3d5
B) [Ar]4s23d3
C) [Ar]4s13d4
D) [Ar]4s24p3
E) [Ar]4p5
A) [Ar]3d5
B) [Ar]4s23d3
C) [Ar]4s13d4
D) [Ar]4s24p3
E) [Ar]4p5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following has the smallest radius?
A) Yb3+
B) Ho3+
C) Nd3+
D) Gd3+
E) Ce3+
A) Yb3+
B) Ho3+
C) Nd3+
D) Gd3+
E) Ce3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
32
The first ionization energy for rubidium is +403.0 kJ/mol. How much energy would be required to convert 17.1 g of gaseous rubidium to its gaseous +1 monatomic ion at constant temperature?
A) 185 kJ
B) 80.6 kJ
C) 68.9 kJ
D) 40.4 kJ
E) 34.5 kJ
A) 185 kJ
B) 80.6 kJ
C) 68.9 kJ
D) 40.4 kJ
E) 34.5 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following has the largest radius?
A) As3-
B) Br
C) Sr2+
D) Cl-
E) Se2-
A) As3-
B) Br
C) Sr2+
D) Cl-
E) Se2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
34
Choose the species from which one electron could most easily be removed.
A) Cl
B) K+
C) K
D) Ca+
E) Ar
A) Cl
B) K+
C) K
D) Ca+
E) Ar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following has the largest radius?
A) Cl-
B) Ar
C) Sc3+
D) K+
E) P3-
A) Cl-
B) Ar
C) Sc3+
D) K+
E) P3-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the general electronic configuration (where n = principal quantum number) that best describes the halogens as a group?
A) ns1np6
B) ns2np4
C) ns2sp5
D) ns2np6
E) ns2np3
A) ns1np6
B) ns2np4
C) ns2sp5
D) ns2np6
E) ns2np3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which series of elements shows the smallest difference in atomic radii?
A) Li......F
B) Be......Ra
C) He......Xe
D) C......Pb
E) Sc......Zn
A) Li......F
B) Be......Ra
C) He......Xe
D) C......Pb
E) Sc......Zn

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which statement best describes relationships in a modern periodic table?
A) Each transition element is placed in the column of the main group element that it most closely resembles.
B) The fourteen elements in the "lanthanide" series form a new independent period.
C) Metalloids usually are the cations in ionic compounds.
D) Nonmetallic properties tend to predominate for elements at the far right portion of the table.
E) Elements are always arranged in order of increasing atomic weights.
A) Each transition element is placed in the column of the main group element that it most closely resembles.
B) The fourteen elements in the "lanthanide" series form a new independent period.
C) Metalloids usually are the cations in ionic compounds.
D) Nonmetallic properties tend to predominate for elements at the far right portion of the table.
E) Elements are always arranged in order of increasing atomic weights.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
39
Among the alkali metals, cesium reacts more rapidly than sodium because:
A) cesium has more electrons
B) cesium has more neutrons
C) the valence electron of cesium is at a greater average distance from the nucleus
D) cesium has a higher atomic weight
E) cesium has more protons
A) cesium has more electrons
B) cesium has more neutrons
C) the valence electron of cesium is at a greater average distance from the nucleus
D) cesium has a higher atomic weight
E) cesium has more protons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which ground state electronic configuration will most readily produce an ion with a charge of 2+?
A) 1s22s22p63s23p63d104s2
B) 1s22s22p63s23p64s1
C) 1s22s22p63s23p4
D) 1s22s22p63s23p63d104s24p2
E) 1s2s22p63s23p63d104s24p6
A) 1s22s22p63s23p63d104s2
B) 1s22s22p63s23p64s1
C) 1s22s22p63s23p4
D) 1s22s22p63s23p63d104s24p2
E) 1s2s22p63s23p63d104s24p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
41
Chlorine has a large negative electron affinity. Therefore, chlorine is most likely to:
A) act as an oxidizing agent
B) have a small first ionization energy
C) act as a reducing agent
D) form a basic oxide
E) release electrons readily to other atoms
A) act as an oxidizing agent
B) have a small first ionization energy
C) act as a reducing agent
D) form a basic oxide
E) release electrons readily to other atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following has the smallest second ionization energy?
A) Si
B) Mg
C) Al
D) P
A) Si
B) Mg
C) Al
D) P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
43
Choose a basic oxide.
A) MgO
B) CO2
C) Cl2O
D) SO2
A) MgO
B) CO2
C) Cl2O
D) SO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following elements form amphoteric oxides?
A) Sn
B) Mg
C) P
D) B
E) Na
A) Sn
B) Mg
C) P
D) B
E) Na

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following species is most likely to be diamagnetic?
A) Fe
B) I
C) Mn+
D) W3+
E) Zn2+
A) Fe
B) I
C) Mn+
D) W3+
E) Zn2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the relationship between ionization energy and atomic radii?
A) Energy increases as radii increase.
B) Energy is negative for all large radii.
C) Energy decreases as radii decrease.
D) Energy decreases as radii increase
E) Energy increases as as radii decrease
A) Energy increases as radii increase.
B) Energy is negative for all large radii.
C) Energy decreases as radii decrease.
D) Energy decreases as radii increase
E) Energy increases as as radii decrease

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the general relationship between electron affinity and atomic radii?
A) Electron affinity becomes more negative as radii increases.
B) Electron affinity becomes more negative as radii decreases.
C) Electron affinity becomes positive as radii decreases.
D) Electron affinity becomes positive as radii increases.
E) The two quantities are unrelated
A) Electron affinity becomes more negative as radii increases.
B) Electron affinity becomes more negative as radii decreases.
C) Electron affinity becomes positive as radii decreases.
D) Electron affinity becomes positive as radii increases.
E) The two quantities are unrelated

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following elements is likely to have the largest second ionization energy?
A) Na
B) Mg
C) Al
D) Si
A) Na
B) Mg
C) Al
D) Si

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
49
Why is the electron affinity so positive for the group 12 elements?
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The groups 12 elements are diatomic elements.
E) Electrons can't be added to gases.
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The groups 12 elements are diatomic elements.
E) Electrons can't be added to gases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following reactions gives a positive value for the electron affinity?
A) S-(g) + e- → S2-(g)
B) O(g) + e- → O-(g)
C) S(g) + e- → S-(g)
D) Br(g) + e- → Br-(g)
A) S-(g) + e- → S2-(g)
B) O(g) + e- → O-(g)
C) S(g) + e- → S-(g)
D) Br(g) + e- → Br-(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
51
The first ionization potential for S is lower than the first ionization potential for P because:
A) Hund's rule is violated
B) P has a p3 configuration
C) ionization potentials decrease across a representative period
D) P is to the right of S on the periodic table
E) P is below S on the periodic table
A) Hund's rule is violated
B) P has a p3 configuration
C) ionization potentials decrease across a representative period
D) P is to the right of S on the periodic table
E) P is below S on the periodic table

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following species is diamagnetic?
A) F-
B) C
C) Al
D) S-
E) Na
A) F-
B) C
C) Al
D) S-
E) Na

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
53
Why is the electron affinity so positive for the noble gas elements?
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The noble gas elements are diatomic elements.
E) Electrons can't be added to gases.
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The noble gas elements are diatomic elements.
E) Electrons can't be added to gases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
54
Can metal atoms form negative ions in the gaseous state? Explain.
A) No, an electron cannot be added.
B) No, only a liquid can be ionized.
C) Yes, electrons can be added to a Li half-filled 2s orbital.
D) Yes, electrons can be removed from a Li half-filled 2s orbital.
E) No, the 2s orbital is completely filled.
A) No, an electron cannot be added.
B) No, only a liquid can be ionized.
C) Yes, electrons can be added to a Li half-filled 2s orbital.
D) Yes, electrons can be removed from a Li half-filled 2s orbital.
E) No, the 2s orbital is completely filled.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which comparison of ionization energies is correct?
A) Cs > Na
B) Na > Mg
C) Ca > Mg
D) Ca+ > Ca
E) Ar > He
A) Cs > Na
B) Na > Mg
C) Ca > Mg
D) Ca+ > Ca
E) Ar > He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following has the greatest number of unpaired electrons?
A) Cr2+
B) Ti3+
C) Co2+
D) Ni
A) Cr2+
B) Ti3+
C) Co2+
D) Ni

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
57
Why is the electron affinity so positive for the group 2 elements?
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The groups 2 elements are diatomic elements.
E) Electrons can't be added to gases.
A) The added electron would have to go into a new shell.
B) The added electron would have to be added into the half-filled p subshell.
C) The added electron would have to be added into the p subshell.
D) The groups 2 elements are diatomic elements.
E) Electrons can't be added to gases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which species is diamagnetic?
A) atomic sodium
B) atomic fluorine
C) iron(III) ion
D) calcium(II) ion
E) cobalt(II) ion
A) atomic sodium
B) atomic fluorine
C) iron(III) ion
D) calcium(II) ion
E) cobalt(II) ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
59
The boiling point for gallium is 2403°C. The boiling point for thallium is 1457°C. Which of the following would be the predicted boiling point for indium?
A) 3860°C
B) 1287°C
C) 946°C
D) 1930°C
E) 730°C
A) 3860°C
B) 1287°C
C) 946°C
D) 1930°C
E) 730°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
60
The first ionization potentials for calcium and barium are 589.7 kJ/mol and 502.8 kJ/mol. What is the predicted first ionization potential for strontium?
A) 546.3 kJ/mol
B) 1092.5 kJ/mol
C) 86.9 kJ/mol
D) 435.7 kJ/mol
E) 588 kJ/mol
A) 546.3 kJ/mol
B) 1092.5 kJ/mol
C) 86.9 kJ/mol
D) 435.7 kJ/mol
E) 588 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the listed elements is the poorest conductor of both heat and electricity?
A) aluminum
B) bismuth
C) cobalt
D) magnesium
E) phosphorus
A) aluminum
B) bismuth
C) cobalt
D) magnesium
E) phosphorus

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which would you expect to have the highest melting point?
A) CF4
B) HI
C) HCl
D) HBr
E) All have the same melting point.
A) CF4
B) HI
C) HCl
D) HBr
E) All have the same melting point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following is an amphoteric oxide?
A) Al2O3
B) Li2O
C) PO3
D) Sc2O3
E) SrO
A) Al2O3
B) Li2O
C) PO3
D) Sc2O3
E) SrO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following has the smallest value of the first ionization energy: Ar, Cs, N, Cu, Rb?
A) Ar
B) Cs
C) N
D) Cu
E) Rb
A) Ar
B) Cs
C) N
D) Cu
E) Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
65
List in order of increasing ionization potential: O, Mg, Rb, S, Si.
A) Si < O < Mg < Rb < S
B) Si < S < Rb < Mg < O
C) Mg < O < Rb < S < Si
D) Rb < Mg < Si < S < O
E) S < Si < Mg < O < Rb
A) Si < O < Mg < Rb < S
B) Si < S < Rb < Mg < O
C) Mg < O < Rb < S < Si
D) Rb < Mg < Si < S < O
E) S < Si < Mg < O < Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
66
List in order of increasing atomic size: F, Na, K, Mg, Ne.
A) K < Mg < Ne < Na < F
B) Ne < Mg < K < Na < F
C) Ne < F < Mg < Na < K
D) Mg < F < Ne < Na < K
E) K < Na < Mg < F < Ne
A) K < Mg < Ne < Na < F
B) Ne < Mg < K < Na < F
C) Ne < F < Mg < Na < K
D) Mg < F < Ne < Na < K
E) K < Na < Mg < F < Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
67
Arrange the following in order of increasing ionization energy: Li, Cs, K.
A) K < Li < Cs
B) Cs < K < Li
C) Li < Cs < K
D) Cs < Li < K
E) K < Cs < Li
A) K < Li < Cs
B) Cs < K < Li
C) Li < Cs < K
D) Cs < Li < K
E) K < Cs < Li

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
68
List in order of increasing ionization energy: Na, K, F, Mg, Ne.
A) Na < K < F < Ne < Mg
B) K < Na < Mg < F < Ne
C) Ne < Mg < F < K < Na
D) F < Mg < Ne < K < Na
E) Ne < Na < Mg < F < K
A) Na < K < F < Ne < Mg
B) K < Na < Mg < F < Ne
C) Ne < Mg < F < K < Na
D) F < Mg < Ne < K < Na
E) Ne < Na < Mg < F < K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following has the highest first ionization potential: Sb, I, In, Xe, Rb?
A) Sb
B) I
C) In
D) Xe
E) Rb
A) Sb
B) I
C) In
D) Xe
E) Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
70
The energy difference between atomic cesium and its most stable ion, in the gaseous state, is referred to as ________.
A) atomic energy
B) electron affinity
C) entropy of vaporization
D) ionization energy
E) magnetic moment
A) atomic energy
B) electron affinity
C) entropy of vaporization
D) ionization energy
E) magnetic moment

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
71
List in order of increasing charge of the most common ion: S, Na, F, Ca, Al
A) S2-, Na-, F+, Ca2+, Al3+
B) Ca2-, F-, Al+, Na2+, S3+
C) F2-, S-, Ca+, Al2+, Na3+
D) S2-, F-, Na+, Ca2+, Al3+
E) Al2-, Ca-, F+, Na2+, S3+
A) S2-, Na-, F+, Ca2+, Al3+
B) Ca2-, F-, Al+, Na2+, S3+
C) F2-, S-, Ca+, Al2+, Na3+
D) S2-, F-, Na+, Ca2+, Al3+
E) Al2-, Ca-, F+, Na2+, S3+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
72
List in order of increasing atomic size: Sr, Se, Cs, Ga, In.
A) Se < Ga < In < Sr < Cs
B) Ga < In < Se < Cs < Sr
C) Sr < In < Ga < Se < Cs
D) In < Ga < Cs < Se < Sr
E) Cs < Ga < Se < Sr < In
A) Se < Ga < In < Sr < Cs
B) Ga < In < Se < Cs < Sr
C) Sr < In < Ga < Se < Cs
D) In < Ga < Cs < Se < Sr
E) Cs < Ga < Se < Sr < In

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
73
If the formula of an oxide is X2O3, what is the formula of the chloride of X?
A) XCl
B) X3Cl
C) XCl3
D) XCl6
E) X2Cl3
A) XCl
B) X3Cl
C) XCl3
D) XCl6
E) X2Cl3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
74
From the following data, what is the estimated boiling point of liquid chlorine? argon/bp 87 K bromine/bp 332 K fluorine/bp 86 K sulfur/bp 718 K
A) 525 K
B) 402 K
C) 209 K
D) 148 K
E) 123 K
A) 525 K
B) 402 K
C) 209 K
D) 148 K
E) 123 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
75
List in order of increasing size: Cl-, Ar, K+, S2-, Ca2+.
A) Ca2+ < K+ < Ar < Cl- < S2-
B) S2- < Cl- < Ar < K+ < Ca2+
C) Ca2+ < S2- < K+ < Ar < Cl-
D) Cl- < Ar < K+ < S2- < Ca2+
E) K+ < S2- < Ar < Cl- < Ca2+
A) Ca2+ < K+ < Ar < Cl- < S2-
B) S2- < Cl- < Ar < K+ < Ca2+
C) Ca2+ < S2- < K+ < Ar < Cl-
D) Cl- < Ar < K+ < S2- < Ca2+
E) K+ < S2- < Ar < Cl- < Ca2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
76
The number of valence electrons for atomic potassium is ________.
A) 1
B) 5
C) 6
D) 15
E) 19
A) 1
B) 5
C) 6
D) 15
E) 19

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which of the following has the highest first ionization potential: K, Ge, Kr, Ca, Br?
A) K
B) Ge
C) Kr
D) Ca
E) Br
A) K
B) Ge
C) Kr
D) Ca
E) Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following atoms has the smallest atomic radius: Br, K, Fe, Cu, Bi?
A) Br
B) K
C) Fe
D) Cu
E) Bi
A) Br
B) K
C) Fe
D) Cu
E) Bi

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which main group atom or ion should be included in the following list? Br-, Se2-, Kr, Sr2+
A) As3-
B) Ar
C) K+
D) S2-
A) As3-
B) Ar
C) K+
D) S2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck
80
List in order of increasing size: K+, Se2-, Cl-, Na+, S2-.
A) Cl- < S2- < K+ < Na+ < Se2-
B) Na+ < K+ < Cl- < S2- < Se2-
C) S2- < Na+ < Cl- < Se2- < K+
D) Cl- < Se2- < K+ < Na+ < S2-
E) K+ < Na+ < Cl- < Se2- < S2-
A) Cl- < S2- < K+ < Na+ < Se2-
B) Na+ < K+ < Cl- < S2- < Se2-
C) S2- < Na+ < Cl- < Se2- < K+
D) Cl- < Se2- < K+ < Na+ < S2-
E) K+ < Na+ < Cl- < Se2- < S2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 96 في هذه المجموعة.
فتح الحزمة
k this deck








