Deck 17: Carboxylic Acids, Esters, and Amides
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/81
العب
ملء الشاشة (f)
Deck 17: Carboxylic Acids, Esters, and Amides
1
What are the products of the reaction shown? 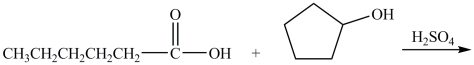
A)hexyl cyclopentanoate + water
B)cyclopentyl hexanoate + water
C)pentyl cyclopentanoate + water
D)cyclopentyl pentanoate + water
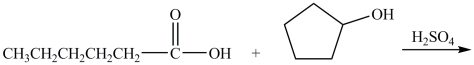
A)hexyl cyclopentanoate + water
B)cyclopentyl hexanoate + water
C)pentyl cyclopentanoate + water
D)cyclopentyl pentanoate + water
B
2
What is the hydrolysis reaction of an ester by a base called?
A)an addition reaction
B)a Fischer esterification
C)a substitution reaction
D)a saponification
A)an addition reaction
B)a Fischer esterification
C)a substitution reaction
D)a saponification
D
3
Which statement is not true?
A)Olestra has properties similar to the triacylglycerols in fats and oils.
B)All natural and synthetic fibers are polymers.
C)Fibers like wool and silk obtained from animals are proteins, and are joined together by many ester linkages.
D)Olestra has so many ester units clustered together that they are too crowded to be hydrolyzed.
A)Olestra has properties similar to the triacylglycerols in fats and oils.
B)All natural and synthetic fibers are polymers.
C)Fibers like wool and silk obtained from animals are proteins, and are joined together by many ester linkages.
D)Olestra has so many ester units clustered together that they are too crowded to be hydrolyzed.
C
4
What products are formed in the base hydrolysis of the ester shown below with NaOH? 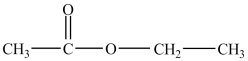
A)ethanol and acetic acid
B)acetic acid and sodium ethoxide
C)methanol and sodium propanoate
D)sodium acetate and water
E)sodium acetate and ethanol
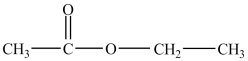
A)ethanol and acetic acid
B)acetic acid and sodium ethoxide
C)methanol and sodium propanoate
D)sodium acetate and water
E)sodium acetate and ethanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which is not a polyamide?
A)Kevlar
B)Nylon
C)Olestra
D)N, N-dimethylpropylamide
E)More than one of the compounds above is not a polyamide.
A)Kevlar
B)Nylon
C)Olestra
D)N, N-dimethylpropylamide
E)More than one of the compounds above is not a polyamide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the IUPAC name of the compound below? 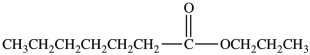
A)heptyl propanoate
B)hexyl propanoate
C)propyl heptanoate
D)4-decanoate
E)propyl hexanoate
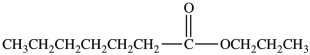
A)heptyl propanoate
B)hexyl propanoate
C)propyl heptanoate
D)4-decanoate
E)propyl hexanoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the IUPAC name of the compound below? 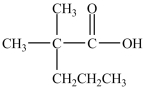
A)2-methyl-2-propylpropanoic acid
B)2-methyl-2-propyl-1-propanoic acid
C)2, 2-dimethylpentanoic acid
D)2, 2-dimethyl-1-pentoic acid
E)4, 4-dimethylpentanoic acid

A)2-methyl-2-propylpropanoic acid
B)2-methyl-2-propyl-1-propanoic acid
C)2, 2-dimethylpentanoic acid
D)2, 2-dimethyl-1-pentoic acid
E)4, 4-dimethylpentanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
8
Heating a carboxylic acid with a primary amine forms water along with what organic product?
A)a primary amide
B)a secondary amide
C)a tertiary amide
D)an ester
A)a primary amide
B)a secondary amide
C)a tertiary amide
D)an ester

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
9
Carboxylic acids react with bases such as NaOH to form what type of compounds?
A)alcohols
B)esters
C)carboxylate salts
D)carboxylic amides
A)alcohols
B)esters
C)carboxylate salts
D)carboxylic amides

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which carboxylic acid is responsible for the sting of some types of ants?
A)formic acid (HCO2H)
B)hexanoic acid (CH3(CH2)4COOH)
C)acetic acid (CH3CO2H)
D)propanoic acid (CH3CH2COOH)
A)formic acid (HCO2H)
B)hexanoic acid (CH3(CH2)4COOH)
C)acetic acid (CH3CO2H)
D)propanoic acid (CH3CH2COOH)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
11
What organic product is formed when a carboxylic acid reacts with an alcohol in the presence of sulfuric acid?
A)an ether
B)an ester
C)a ketone
D)an amide
A)an ether
B)an ester
C)a ketone
D)an amide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following properly describes soaps?
A)fatty acids
B)salts of carboxylic acids that have a long hydrocarbon chain
C)salts of carboxylic acids that have a short hydrocarbon chain
D)carboxylic acids
A)fatty acids
B)salts of carboxylic acids that have a long hydrocarbon chain
C)salts of carboxylic acids that have a short hydrocarbon chain
D)carboxylic acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the IUPAC name of the compound below? 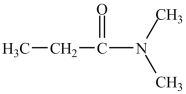
A)N, N-dimethylpropanamide
B)N, N-dimethylethylamide
C)dimethylpropanamide
D)dimethylaminopropanamide
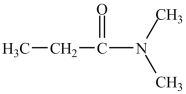
A)N, N-dimethylpropanamide
B)N, N-dimethylethylamide
C)dimethylpropanamide
D)dimethylaminopropanamide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
14
What products are formed in the acid hydrolysis of the ester shown below? 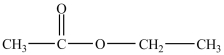
A)ethanol and acetic acid
B)acetic acid and water
C)propanoic acid and ethanol
D)acetic acid and ethane
E)ethanal and ethanol
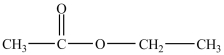
A)ethanol and acetic acid
B)acetic acid and water
C)propanoic acid and ethanol
D)acetic acid and ethane
E)ethanal and ethanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which statement about the labeled carbons in the compound below is true? 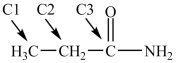
A)C1 is an carbon and C2 is a carbon
B)C2 is an carbon and C1 is a carbon
C)C3 is an carbon and C2 is a carbon
D)C2 is an carbon and C3 is a carbon
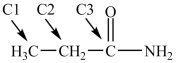
A)C1 is an carbon and C2 is a carbon
B)C2 is an carbon and C1 is a carbon
C)C3 is an carbon and C2 is a carbon
D)C2 is an carbon and C3 is a carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which amide has the lowest boiling point?
A)CH3(CH2)5CONH2
B)CH3CH2CON(CH2CH3)2
C)CH3CH2CH2CH2CONHCH2CH3
D)All of the amides above have the same boiling point.
A)CH3(CH2)5CONH2
B)CH3CH2CON(CH2CH3)2
C)CH3CH2CH2CH2CONHCH2CH3
D)All of the amides above have the same boiling point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the IUPAC name of the compound below? 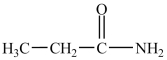
A)propanamide
B)ethylamide
C)propanamine
D)N-propylamide
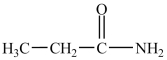
A)propanamide
B)ethylamide
C)propanamine
D)N-propylamide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the common name of the compound below? 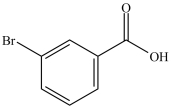
A)m-bromobenzoic acid
B)3-bromoacetobenzenoic acid
C)o-bromobenzene carboxylic acid
D)3-bromo-1-acetobenzoic acid

A)m-bromobenzoic acid
B)3-bromoacetobenzenoic acid
C)o-bromobenzene carboxylic acid
D)3-bromo-1-acetobenzoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
19
The reaction of a carboxylic acid (RCOOH)with an alcohol (R'OH)in the presence of an acid catalyst to form an ester (RCOOR')is called which of the following?
A)an addition reaction
B)a Fischer esterification
C)an acid-base reaction
D)a saponification
A)an addition reaction
B)a Fischer esterification
C)an acid-base reaction
D)a saponification

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which compound has the highest boiling point?
A)(CH3)2CHCH2CHO
B)CH3CH2CH2CH2CH2COOH
C)(CH3)2CHCH2CH2CH2OH
D)CH3(CH2)6CH3
E)All of the compounds above have the same boiling point.
A)(CH3)2CHCH2CHO
B)CH3CH2CH2CH2CH2COOH
C)(CH3)2CHCH2CH2CH2OH
D)CH3(CH2)6CH3
E)All of the compounds above have the same boiling point.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
21
The compound below is named potassium 2-chloro-3-hydroxybutanoate. 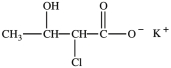


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which statement is false?
A)Aspirin is a synthetic compound; that is, it does not occur in nature.
B)The active ingredient in aspirin is acetylsalicylic acid.
C)Salicin, found in willow bark, and salicylic acid, found in meadowsweet blossoms, have structures that are similar to aspirin.
D)Aspirin is a strong acid.
A)Aspirin is a synthetic compound; that is, it does not occur in nature.
B)The active ingredient in aspirin is acetylsalicylic acid.
C)Salicin, found in willow bark, and salicylic acid, found in meadowsweet blossoms, have structures that are similar to aspirin.
D)Aspirin is a strong acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
23
When the compound below reacts with water in the presence of HCl, ethanol and acetic acid are formed. 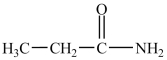
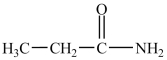

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
24
When soap is mixed with water, the nonpolar hydrocarbon tails dissolve the dirt on the exterior of micelles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
25
To be active, aspirin must cross a cell membrane, and to do so, it must be ionic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
26
Clavulanic acid (structure shown)is a drug used in conjunction with some antibiotics to overcome certain types of antibiotic resistance. How many chirality centers are in clavulanic acid? 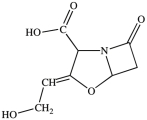
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
27
Clavulanic acid (structure shown)is a drug used in conjunction with some antibiotics to overcome certain types of antibiotic resistance. How many chirality centers are in clavulanic acid? 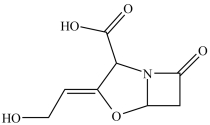
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
28
Animal fats and vegetable oils are composed of triacylglycerols.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of these structures represents a soap?
A)CH3CO2- K+
B)CH3(CH2)14CO2- Na+
C)CH3(CH2)12COOH
D)CH3(CH2)7CO2(CH2)7Na
E)More than one of the compounds above is a soap.
A)CH3CO2- K+
B)CH3(CH2)14CO2- Na+
C)CH3(CH2)12COOH
D)CH3(CH2)7CO2(CH2)7Na
E)More than one of the compounds above is a soap.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
30
Aspirin relieves pain and decreases inflammation because it prevents the synthesis of prostaglandins, the compounds responsible for both of these physiological responses.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
31
Triacylglycerols are esters formed from glycerol and three acids, each of which has a long carbon chain bonded to the carbonyl group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which name is possible for a carboxylic acid?
A)1-chloropentanoic acid
B)2-pentylbutanoic acid
C)m-butylbenzoic acid
D)6, 6-dimethylhexanoic acid
A)1-chloropentanoic acid
B)2-pentylbutanoic acid
C)m-butylbenzoic acid
D)6, 6-dimethylhexanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
33
Clavulanic acid (structure shown)is a drug used in conjunction with some antibiotics to overcome certain types of antibiotic resistance. Which of these functional groups is NOT contained in clavulanic acid? 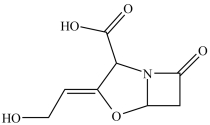
A)carboxylic acid
B) -lactam
C)lactone
D)ether

A)carboxylic acid
B) -lactam
C)lactone
D)ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
34
The compound below can hydrogen bond to another molecule like itself. 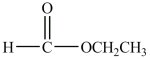
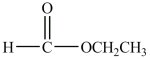

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
35
Treatment of an amide (RCONHR')with water in the presence of an acid catalyst (HCl)forms a carboxylic acid with the structure R'COOH, and an amine salt with the structure RNH3+ Cl-.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which compound is the most soluble in water?
A)(CH3)2CHCH2COOCH3
B)CH3CH2CH2CH2CH2COOH
C)CH3(CH2)6CH3
D)CH3CH2CH2CH2CH2COOK
A)(CH3)2CHCH2COOCH3
B)CH3CH2CH2CH2CH2COOH
C)CH3(CH2)6CH3
D)CH3CH2CH2CH2CH2COOK

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
37
A common type of reaction for acyl compounds is substitution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
38
The compound below is named potassium 2-chloro-3-hydroxybutanoate. 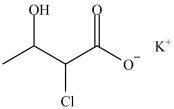


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
39
Fischer esterification can be used to synthesize aspirin from salicylic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
40
Clavulanic acid (structure shown)is a drug used in conjunction with some antibiotics to overcome certain types of antibiotic resistance. Which of these functional groups is NOT contained in clavulanic acid? 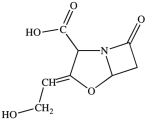
A)carboxylic acid
B) -lactam
C)lactone
D)ether

A)carboxylic acid
B) -lactam
C)lactone
D)ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
41
The compound 3-chloro-2-hydroxybutanoic acid is an -hydroxy acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
42
Nylon is an example of a condensation polymer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
43
Compounds that contain both a hydroxyl group (OH)and a carboxyl group (COOH)can undergo an intramolecular esterification reaction. The lactone product shown is the result of the intramolecular esterification reaction of the indicated starting material. 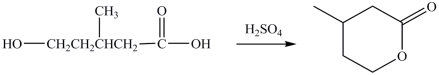
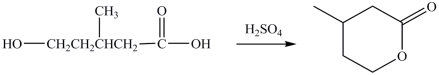

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
44
Penicillin acts as an antibiotic by reacting with an enzyme needed to synthesize the cell nucleus of a bacterium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
45
The first step in the metabolism of a triacylglycerol is hydrolysis of the ester bonds to form glycerol and three fatty acids in a reaction called a simple ester hydrolysis. In the cells of the body, this reaction is catalyzed by acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
46
A lactam can be a primary amide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
47
The ester below contains a propoxy group. 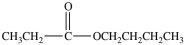
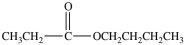

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
48
Polyesters are condensation polymers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
49
The two compounds below have approximately the same boiling point. 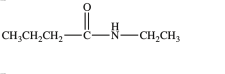


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
50
Compounds that contain both a hydroxyl group (OH)and a carboxyl group (COOH)can undergo an intramolecular esterification reaction. The lactone product shown is the result of the intramolecular esterification reaction of the indicated starting material. 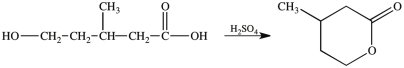


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
51
All natural and synthetic fibers are polymers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
52
The compound below is an example of a lactam. 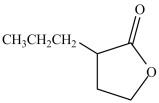


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
53
The compound below has the molecular formula C9H11NO, contains a benzene ring, and is a 2° amide. 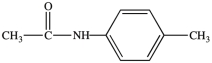


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
54
A condensation polymer is a polymer formed when monomers containing two functional groups come together with the gain of a small molecule such as water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
55
The compound below has the molecular formula C9H11NO, contains a benzene ring, and is a 2° amide. 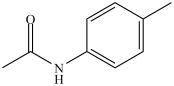


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
56
The ester below contains a propoxy group. 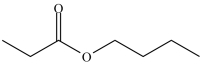


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
57
The salts of carboxylic acids are commonly used as preservatives.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
58
When a carboxylic acid is dissolved in water, an acid-base equilibrium occurs: the carboxylic acid donates a proton to H2O, forming its conjugate base, a carboxylate anion, and water gains a proton, forming its conjugate acid, H3O+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
59
The two compounds below have approximately the same boiling point. 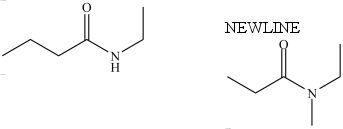


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
60
The polyamide product shown is the result of the polymerization reaction between the indicated diacid and amine. 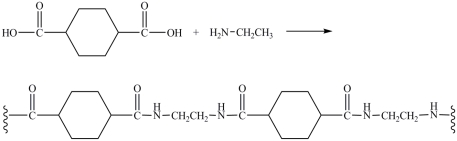


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
61
All carbonyl compounds that contain a nitrogen atom are amides.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
62
The psychoactive drug benzodiazepine (structure shown)contains two amide groups. 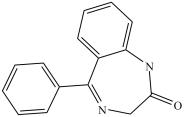


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
63
Carboxylic acids are capable of hydrogen bonding and often form dimers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
64
(CH3)2CHCH2OH has a lower boiling point than CH3CH2COOH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
65
The IUPAC name of the compound below is _____. 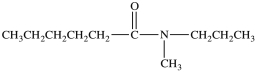


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
66
_____ is the common name of the simplest amide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
67
In a propanoic acid dimer, the two propanoic acid molecules are held together by two _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
68
The name of the compound below is N-ethylpentanamide. 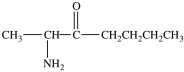


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
69
The name of the compound below is N-ethylpentanamide. 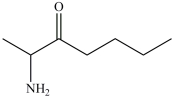


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
70
A tertiary amide contains _____ C-N bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
71
A cyclic ester is called a(n)_____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
72
Carboxylic acids, esters, and amides are three families of organic molecules that contain a carbonyl group (C=O)bonded to an element more electronegative than carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
73
The IUPAC name of the compound below is _____. 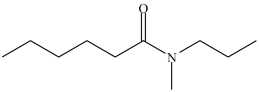


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
74
Carboxylic acids, esters, and amides are called _____ compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
75
(CH3)2CHCHO has a lower boiling point than CH3CH2CH2CH2COOH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
76
Dissolving soap in water forms spherical droplets called _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
77
The two compounds shown below are formed when N-butylacetamide is hydrolyzed with water in the presence of NaOH. 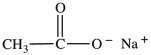 and
and 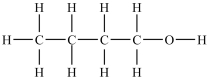
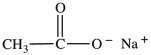 and
and 

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
78
Tertiary (3°)amides have higher boiling points and melting points than primary (1°)and secondary (2°)amides.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
79
When a carboxylic acid is dissolved in water, an acid-base equilibrium occurs: the carboxylic acid donates a proton to H2O, forming its conjugate base, a _____, and water gains a proton, forming its conjugate acid, H3O+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
80
When trimethylamine is heated up with propanoic acid, an amide is formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck








