Deck 12: Alkanes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/106
العب
ملء الشاشة (f)
Deck 12: Alkanes
1
What is the IUPAC name of this compound? 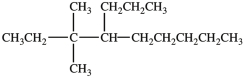
A)4-isopentylnonane
B)3-ethyl-4-propylnonane
C)3, 3-dimethyl-4-propylnonane
D)3, 3-dimethyl-4-butylnonane
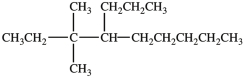
A)4-isopentylnonane
B)3-ethyl-4-propylnonane
C)3, 3-dimethyl-4-propylnonane
D)3, 3-dimethyl-4-butylnonane
C
2
What is the IUPAC name of CH3CH2CH2CH2CH2CH3?
A)butane
B)hexane
C)propane
D)pentane
E)heptane
A)butane
B)hexane
C)propane
D)pentane
E)heptane
B
3
What is the IUPAC name of this compound? 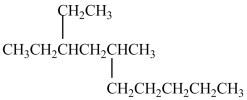
A)3-ethyl-5-methyldecane
B)3-ethyl-2-pentylhexane
C)3-ethyl-5-pentylhexane
D)8-ethyl-6-methyldecane
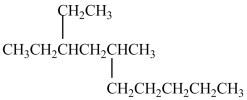
A)3-ethyl-5-methyldecane
B)3-ethyl-2-pentylhexane
C)3-ethyl-5-pentylhexane
D)8-ethyl-6-methyldecane
A
4
What is the IUPAC name of this compound? 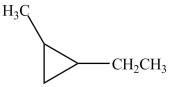
A)ethyl-methyl-cyclopropane
B)ethyl-2-methylcyclobutane
C)1-ethyl-2-methylcyclobutane
D)1-ethyl-2-methylcyclopropane
E)ethyl-2-methylcyclotriane

A)ethyl-methyl-cyclopropane
B)ethyl-2-methylcyclobutane
C)1-ethyl-2-methylcyclobutane
D)1-ethyl-2-methylcyclopropane
E)ethyl-2-methylcyclotriane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which formula represents a cyclic alkane?
A)C10H18
B)C10H22
C)C9H20
D)C10H20
E)C11H20
A)C10H18
B)C10H22
C)C9H20
D)C10H20
E)C11H20

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
6
How many hydrogen atoms are present in a cyclic alkane with six (6)carbon atoms?
A)6
B)8
C)10
D)12
E)14
A)6
B)8
C)10
D)12
E)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which formula represents an acyclic alkane?
A)C10H18
B)C9H18
C)C12H26
D)C10H20
E)C12H28
A)C10H18
B)C9H18
C)C12H26
D)C10H20
E)C12H28

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which compound is a constitutional isomer of the one shown below? 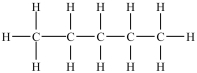
A)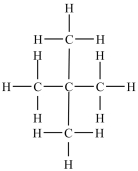
B)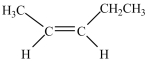
C)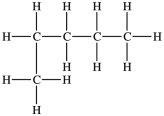
D)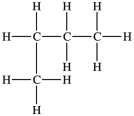
E)More than one of the compounds above is a constitutional isomer.

A)

B)

C)

D)

E)More than one of the compounds above is a constitutional isomer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which structure depicts an alkane of molecular formula C6H12 that has two 1° carbons and two 3° carbons?
A)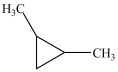
B)CH3CH2CH2CH2CH2CH3
C)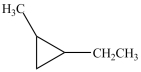
D)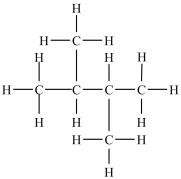
A)

B)CH3CH2CH2CH2CH2CH3
C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the most preferred arrangement of the atoms in n-pentane?
A)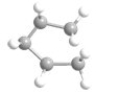
B)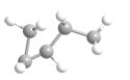
C)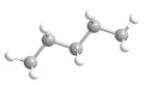
D)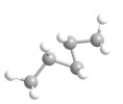
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the IUPAC name of this compound? 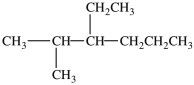
A)4-propylhexane
B)3-ethyl-2-methylhexane
C)2-methyl-3-ethylhexane
D)4-ethyl-5-methylheptane

A)4-propylhexane
B)3-ethyl-2-methylhexane
C)2-methyl-3-ethylhexane
D)4-ethyl-5-methylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many hydrogen atoms are present in an acyclic alkane with five (5)carbon atoms?
A)5
B)7
C)9
D)10
E)12
A)5
B)7
C)9
D)10
E)12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many secondary carbons are in the straight-chain alkane with the formula C4H10?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is a constitutional isomer of the compound shown below? 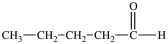
A)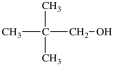
B)
C)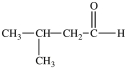
D)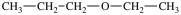
E)More than one of the molecules above is a constitutional isomer of the original molecule.
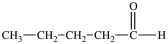
A)

B)

C)

D)
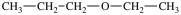
E)More than one of the molecules above is a constitutional isomer of the original molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following is a constitutional isomer of the compound shown below? 
A)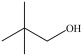
B)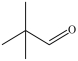
C)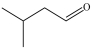
D)
E)More than one of the molecules above is a constitutional isomer of the original molecule.

A)

B)

C)

D)

E)More than one of the molecules above is a constitutional isomer of the original molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the classification of each labeled carbon atom in the structure below? 
A)C1 is a secondary carbon and C2 is a primary carbon.
B)C1 is a tertiary carbon and C2 is a secondary carbon.
C)C1 is a secondary carbon and C2 is a tertiary carbon.
D)C1 is a primary carbon and C2 is a tertiary carbon.

A)C1 is a secondary carbon and C2 is a primary carbon.
B)C1 is a tertiary carbon and C2 is a secondary carbon.
C)C1 is a secondary carbon and C2 is a tertiary carbon.
D)C1 is a primary carbon and C2 is a tertiary carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following compounds is identical to the one shown below? 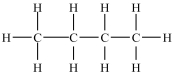
A)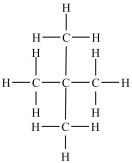
B)
C)
D)

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
18
How many primary carbon atoms are in the straight-chain alkane with the formula C3H8?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the IUPAC name of CH3CH2CH2CH3?
A)butane
B)methane
C)propane
D)pentane
A)butane
B)methane
C)propane
D)pentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the IUPAC name of this compound? 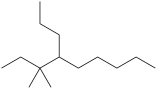
A)4-isopentylnonane
B)3-ethyl-4-propylnonane
C)3, 3-dimethyl-4-propylnonane
D)3, 3-dimethyl-4-butylnonane

A)4-isopentylnonane
B)3-ethyl-4-propylnonane
C)3, 3-dimethyl-4-propylnonane
D)3, 3-dimethyl-4-butylnonane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the structure of 2-methylhexane?
A)(CH3)2CHCH2CH2CH3
B)(CH3)2CHCH2CH2CH2CH3
C)(CH3)3CCH2CH2CH2CH3
D)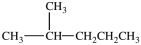
A)(CH3)2CHCH2CH2CH3
B)(CH3)2CHCH2CH2CH2CH3
C)(CH3)3CCH2CH2CH2CH3
D)
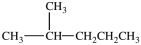

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which compound is identical to this molecule? 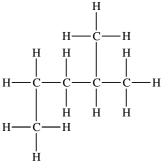
A)(CH3)2CHCH2CH2CH3
B)CH3(CH2)4CH3
C)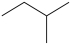
D)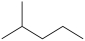
E)More than one molecule is identical to the original molecule.

A)(CH3)2CHCH2CH2CH3
B)CH3(CH2)4CH3
C)

D)

E)More than one molecule is identical to the original molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which name represents a valid IUPAC name for an alkane?
A)1, 4-diethylheptane
B)4, 4-diethyl-2, 3, 4-trimethylpentane
C)4, 4-diethyl-2, 3, 4-trimethylcycloheptane
D)4, 4-diethyl-2, 3-dimethylheptane
E)More than one of the names above is a valid IUPAC name for an alkane.
A)1, 4-diethylheptane
B)4, 4-diethyl-2, 3, 4-trimethylpentane
C)4, 4-diethyl-2, 3, 4-trimethylcycloheptane
D)4, 4-diethyl-2, 3-dimethylheptane
E)More than one of the names above is a valid IUPAC name for an alkane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which has the highest boiling point?
A)natural gas
B)lubricating oil
C)gasoline
D)kerosene
E)fuel oil
A)natural gas
B)lubricating oil
C)gasoline
D)kerosene
E)fuel oil

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which balanced chemical equation represents the incomplete combustion of a hydrocarbon?
A)CH3CH2CH3 + 5 O2 3 CO2 + 4 H2O
B)2 CH3CH2CH3 + 7 O2 6 CO + 8 H2O
C)C36H74 + 36 O2 36 CO + 37 H2O
D)C26H50 + 33 O2 16 CO2 + 10 CO + 25 H2O
A)CH3CH2CH3 + 5 O2 3 CO2 + 4 H2O
B)2 CH3CH2CH3 + 7 O2 6 CO + 8 H2O
C)C36H74 + 36 O2 36 CO + 37 H2O
D)C26H50 + 33 O2 16 CO2 + 10 CO + 25 H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which name is not a valid IUPAC name for an alkane?
A)2, 3, 4-trimethylhexane
B)1-butyl-2-ethylcyclopentane
C)1-ethyl-2-butylpentane
D)5-butyl-2-methylnonane
A)2, 3, 4-trimethylhexane
B)1-butyl-2-ethylcyclopentane
C)1-ethyl-2-butylpentane
D)5-butyl-2-methylnonane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the structure of butylcyclopentane?
A)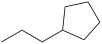
B)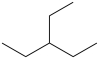
C)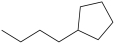
D)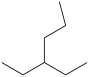
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which molecule is a constitutional isomer of the compound (CH3)2CH(CH2)2CH3?
A)CH3CH2CH2CH(CH3)2
B)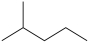
C)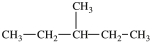
D)CH3CH2CH2CH2CH3
A)CH3CH2CH2CH(CH3)2
B)

C)

D)CH3CH2CH2CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the classification of each labeled carbon atom in this structure? 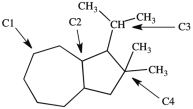
A)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 4°.
B)C1 is a 2°, C2 is 3°, C3 is a 3°, and C4 is 4°.
C)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 1°.
D)C1 is a 2°, C2 is 3°, C3 is a 2°, and C4 is 3°.

A)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 4°.
B)C1 is a 2°, C2 is 3°, C3 is a 3°, and C4 is 4°.
C)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 1°.
D)C1 is a 2°, C2 is 3°, C3 is a 2°, and C4 is 3°.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which alkane has the lowest boiling point?
A)octane
B)butane
C)ethane
D)hexane
E)propane
A)octane
B)butane
C)ethane
D)hexane
E)propane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the IUPAC name of this molecule? 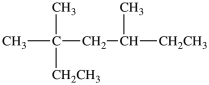
A)2, 4-dimethyl-2-ethylhexane
B)2-ethyl-2, 4-dimethylhexane
C)3, 3, 5-trimethylheptane
D)5-ethyl-3, 5-dimethylhexane

A)2, 4-dimethyl-2-ethylhexane
B)2-ethyl-2, 4-dimethylhexane
C)3, 3, 5-trimethylheptane
D)5-ethyl-3, 5-dimethylhexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
32
How many secondary carbons are in the molecule (CH3)2CH(CH2)2CH3?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which compound has the lowest melting point?
A)CH3CH2CH2CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
A)CH3CH2CH2CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the structure of 2-methylhexane?
A)(CH3)2CHCH2CH2CH3
B)(CH3)2CHCH2CH2CH2CH3
C)(CH3)3CCH2CH2CH2CH3
D)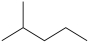
A)(CH3)2CHCH2CH2CH3
B)(CH3)2CHCH2CH2CH2CH3
C)(CH3)3CCH2CH2CH2CH3
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many tertiary carbons are in the molecule (CH3)2CH(CH2)2CH3?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the structure of butylcyclopentane?
A)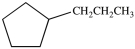
B)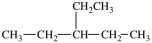
C)
D)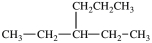
A)

B)

C)

D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many quaternary carbons are in this molecule? 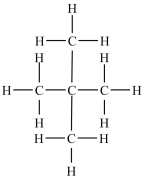
A)0
B)1
C)4
D)5

A)0
B)1
C)4
D)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which molecule is a constitutional isomer of the compound (CH3)2CH(CH2)2CH3?
A)CH3CH2CH2CH(CH3)2
B)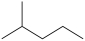
C)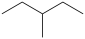
D)
A)CH3CH2CH2CH(CH3)2
B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the classification of each labeled carbon atom in this structure? 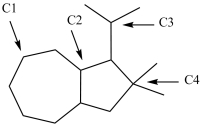
A)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 4°.
B)C1 is a 2°, C2 is 3°, C3 is a 3°, and C4 is 4°.
C)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 1°.
D)C1 is a 2°, C2 is 3°, C3 is a 2°, and C4 is 3°.

A)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 4°.
B)C1 is a 2°, C2 is 3°, C3 is a 3°, and C4 is 4°.
C)C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 1°.
D)C1 is a 2°, C2 is 3°, C3 is a 2°, and C4 is 3°.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
40
How many polar bonds are in this molecule? 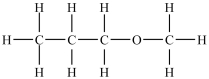
A)0
B)1
C)2
D)14

A)0
B)1
C)2
D)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
41
Asphalt is a mixture of high molecular weight hydrocarbons. Which solvent would be best for removing asphalt from a newly paved road off the bottom of a pair of shoes?
A)water
B)octane
C)methanol, CH3OH
D)NaCl(aq)
A)water
B)octane
C)methanol, CH3OH
D)NaCl(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
42
The combustion of alkanes and other hydrocarbons obtained from fossil fuels adds a tremendous amount of CO2 to the atmosphere each year.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
43
CO2 is a greenhouse gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following pairs of compounds are constitutional isomers?
A)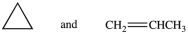
B)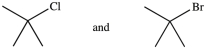
C)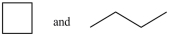
D)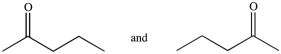
A)
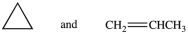
B)
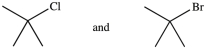
C)
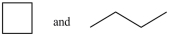
D)
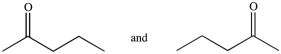

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
45
The products of the complete combustion of any hydrocarbon are CO2 + H2O, regardless of the identity of the starting material.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
46
Eicosane has the structure: CH3(CH2)18CH3. What does the prefix eicos specifically indicate about the structure of this compound?
A)It contains a straight chain of carbons.
B)It contains 20 carbons in a continuous chain.
C)It is an alkane.
D)It is a saturated hydrocarbon.
A)It contains a straight chain of carbons.
B)It contains 20 carbons in a continuous chain.
C)It is an alkane.
D)It is a saturated hydrocarbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
47
The IUPAC name of the molecule (CH3)2CH(CH2)2CH3 is 2-methylhexane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which is NOT a property associated with tetradecane, a 14-carbon straight chain alkane?
A)strong hydrogen bonds
B)low water solubility
C)density less than water
D)flammable liquid
A)strong hydrogen bonds
B)low water solubility
C)density less than water
D)flammable liquid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which molecular formula has the largest number of constitutional isomers?
A)C5H12
B)C2H6
C)C6H14
D)All of the molecular formulas above have the same number of constitutional isomers.
A)C5H12
B)C2H6
C)C6H14
D)All of the molecular formulas above have the same number of constitutional isomers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of the following pairs of compounds are constitutional isomers?
A)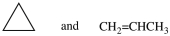
B)(CH3)3CCl and (CH3)3CBr
C)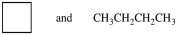
D)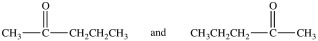
A)
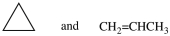
B)(CH3)3CCl and (CH3)3CBr
C)
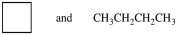
D)
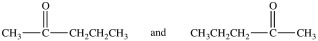

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
51
Alkanes containing only secondary carbon atoms do not exist.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
52
A branched alkane and a cycloalkane with the same number of carbons have the same number of hydrogen atoms in their chemical formulas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the classification of the carbon atom that bonded to the -OH group in menthol? 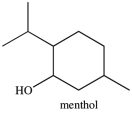
A)primary
B)secondary
C)tertiary
D)quaternary

A)primary
B)secondary
C)tertiary
D)quaternary

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
54
All organic molecules must have at least one primary carbon atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
55
Branching in an alkane chain decreases surface area. With this in mind, which of the following alkanes has the highest boiling point?
A)(CH3)2CHCH2CH2CH2CH2CH3
B)(CH3)2CHCH2CH2CH(CH3)2
C)CH3CH2CH2CH2CH2CH2CH2CH3
D)(CH3)3CC(CH3)3
A)(CH3)2CHCH2CH2CH2CH2CH3
B)(CH3)2CHCH2CH2CH(CH3)2
C)CH3CH2CH2CH2CH2CH2CH2CH3
D)(CH3)3CC(CH3)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
56
Shark liver oil is approximately 14% pristane (C19H40). Pristane is an acyclic alkane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which compound is identical to this molecule? 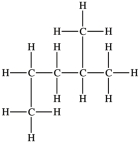
A)(CH3)2CHCH2CH2CH3
B)CH3(CH2)4CH3
C)CH3CH2CH(CH3)2
D)CH3(CH2)2CH(CH3)2
E)More than one molecule is identical to the original molecule.

A)(CH3)2CHCH2CH2CH3
B)CH3(CH2)4CH3
C)CH3CH2CH(CH3)2
D)CH3(CH2)2CH(CH3)2
E)More than one molecule is identical to the original molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
58
The molecules below are constitutional isomers. 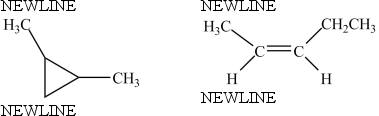


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
59
The compounds below are constitutional isomers. 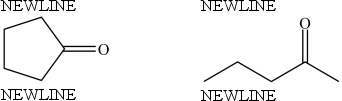


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the classification of the carbon atom that bonded to the -OH group in menthol? 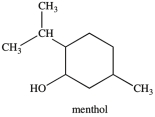
A)primary
B)secondary
C)tertiary
D)quaternary

A)primary
B)secondary
C)tertiary
D)quaternary

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
61
The compounds below are constitutional isomers. 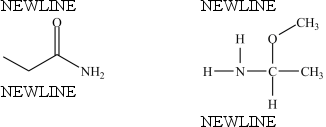


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
62
If a cyclic alkane has 28 hydrogens and a single ring, it must have 13 carbon atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
63
The compounds below are identical. 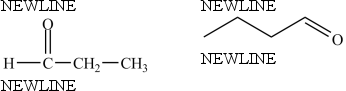
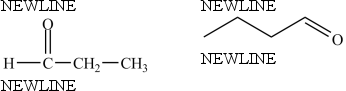

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
64
Cyclic alkanes are planar molecules containing one or more rings of carbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
65
The two structures below represent the same compound. 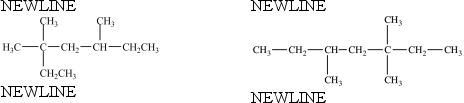


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
66
The two structures below represent the same compound. 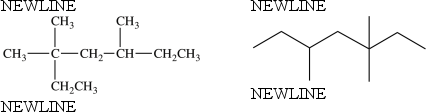


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
67
The IUPAC name of the molecule below is butylcyclopentane. 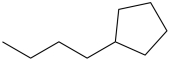


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
68
The IUPAC name of the molecule below is butylcyclopentane. 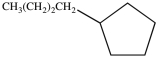


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
69
The compounds below are identical. 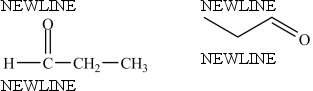
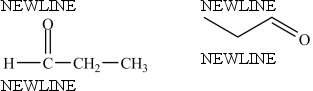

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
70
C2H6 has a higher boiling point than the compound below. 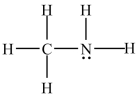


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
71
The molecular formula for an alkane with 12 carbon atoms and two rings is C12H24.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
72
The molecule below contains 1°, 2°, and 3°carbon atoms, but no 4° carbon atoms. 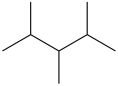


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
73
Natural gas is composed primarily of propane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
74
The compounds below are constitutional isomers. 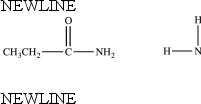


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
75
Pentane has a higher boiling point than 2, 2-dimethylpropane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
76
Oxidation results in a decrease in the number of C-O bonds and an increase in the number of C-H bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
77
The compounds below are constitutional isomers. 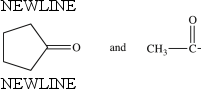


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
78
The compounds below are constitutional isomers. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
79
Acyclic alkanes with four or more carbon atoms have constitutional isomers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck
80
The compounds below are constitutional isomers. 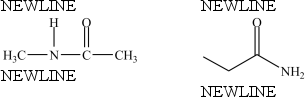
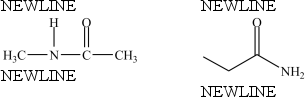

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 106 في هذه المجموعة.
فتح الحزمة
k this deck








