Deck 9: Acids and Bases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/90
العب
ملء الشاشة (f)
Deck 9: Acids and Bases
1
An Arrhenius acid is
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
D
2
Which species is the conjugate base of NH3?
A)NH4+
B)NH2
C)NH2-
D)H2O
E)NH4OH
A)NH4+
B)NH2
C)NH2-
D)H2O
E)NH4OH
C
3
In the acid-base reaction: HCN(aq)+ F-(aq) 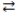 CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
A)products are favored.
B)reactants are favored.
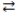 CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, theA)products are favored.
B)reactants are favored.
B
4
Which species can act as a Brønsted-Lowry acid?
A)CO32-
B)HBr
C)Br2
D)LiOH
A)CO32-
B)HBr
C)Br2
D)LiOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which ion is the strongest base?
A)Br-
B)F-
C)I-
D)NO3-
A)Br-
B)F-
C)I-
D)NO3-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
6
A Brønsted-Lowry acid is
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which acid is the weakest?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which species is the conjugate acid of HCO3-?
A)CO32-
B)H2CO3
C)CO2
D)H2O
A)CO32-
B)H2CO3
C)CO2
D)H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which compound is a weak acid?
A)HNO3
B)HBr
C)CH3COOH
D)H2SO4
A)HNO3
B)HBr
C)CH3COOH
D)H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which species is a diprotic acid?
A)Mg(OH)2
B)CH3COOH
C)H2
D)H2CO3
A)Mg(OH)2
B)CH3COOH
C)H2
D)H2CO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which solution has the highest pH?
A)4.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M -OH
E)1.0 × 10-2 M H3O+
A)4.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M -OH
E)1.0 × 10-2 M H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which acid has the strongest conjugate base?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which species can act as a Brønsted-Lowry base?
A)CO32-
B)HBr
C)H2CO3
D)NH4+
A)CO32-
B)HBr
C)H2CO3
D)NH4+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which acid is the strongest?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
15
The [H3O+] in a cabernet sauvignon wine is 5.9 × 10-4 M. What is the [-OH] in this wine?
A)5.9 × 10-4 M -OH
B)1.0 × 10-7 M -OH
C)5.9 × 10-18 M -OH
D)1.7 × 10-11 M -OH
E)1.0 × 10-14 M -OH
A)5.9 × 10-4 M -OH
B)1.0 × 10-7 M -OH
C)5.9 × 10-18 M -OH
D)1.7 × 10-11 M -OH
E)1.0 × 10-14 M -OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
16
In the acid-base reaction: F-(aq)+ HNO3(aq) 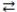 HF(aq)+ NO3-(aq)
HF(aq)+ NO3-(aq)
A)F- is the acid and its conjugate base is HF, and HNO3 is the base and its conjugate acid is NO3-(aq).
B)HNO3 is the acid and its conjugate base is NO3-(aq), and F- is the base and its conjugate acid is HF.
C)F- is the acid and its conjugate base is NO3-(aq), and HNO3 is the base and its conjugate acid is HF.
D)HNO3 is the acid and its conjugate base is HF, and F- is the base and its conjugate acid is NO3-(aq).
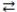 HF(aq)+ NO3-(aq)
HF(aq)+ NO3-(aq)A)F- is the acid and its conjugate base is HF, and HNO3 is the base and its conjugate acid is NO3-(aq).
B)HNO3 is the acid and its conjugate base is NO3-(aq), and F- is the base and its conjugate acid is HF.
C)F- is the acid and its conjugate base is NO3-(aq), and HNO3 is the base and its conjugate acid is HF.
D)HNO3 is the acid and its conjugate base is HF, and F- is the base and its conjugate acid is NO3-(aq).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which solution has the highest pH?
A)4.3 × 10-8 M H3O+
B)1.0 × 10-7 M H3O+
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M H3O+
E)1.0 × 10-2 M H3O+
A)4.3 × 10-8 M H3O+
B)1.0 × 10-7 M H3O+
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M H3O+
E)1.0 × 10-2 M H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
18
The [-OH] in a sample of egg whites is 6.3 × 10-7 M. What is the [H3O+] in these egg whites?
A)6.3 × 10-7 M H3O+
B)1.0 × 10-7 M H3O+
C)6.3 × 10-21 M H3O+
D)1.6 × 10-8 M H3O+
E)1.0 × 10-14 M H3O+
A)6.3 × 10-7 M H3O+
B)1.0 × 10-7 M H3O+
C)6.3 × 10-21 M H3O+
D)1.6 × 10-8 M H3O+
E)1.0 × 10-14 M H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
19
Ammonia, NH3, is an example of a
A)strong acid
B)strong base
C)weak acid
D)weak base
A)strong acid
B)strong base
C)weak acid
D)weak base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the expression for Kw, the ion-product constant for water?
A)Kw = [H2O]2
B)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_9896_9547_45ea193798ec_TB5866_11.jpg)
C)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_9897_9547_2da9c4e98288_TB5866_11.jpg)
D)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_bfa8_9547_ebf851b48095_TB5866_11.jpg)
A)Kw = [H2O]2
B)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_9896_9547_45ea193798ec_TB5866_11.jpg)
C)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_9897_9547_2da9c4e98288_TB5866_11.jpg)
D)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de2f_bfa8_9547_ebf851b48095_TB5866_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the pH of a cleaning solution with a [H3O+] = 7.4 × 10-9 M H3O+?
A)5.9
B)7.13
C)8.13
D)5.87
A)5.9
B)7.13
C)8.13
D)5.87

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which buffer solution has the lowest pH (HF has Ka = 7.2 × 10-4)?
A)0.10 M HF and 0.10 M NaF
B)0.20 M HF and 0.20 M NaF
C)0.20 M HF and 0.10 M NaF
D)0.10 M HF and 0.20 M NaF
E)All of the buffer solutions described above have the same pH.
A)0.10 M HF and 0.10 M NaF
B)0.20 M HF and 0.20 M NaF
C)0.20 M HF and 0.10 M NaF
D)0.10 M HF and 0.20 M NaF
E)All of the buffer solutions described above have the same pH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which salt forms a solution with a pH < 7 when dissolved in water?
A)RbI
B)NaCH3COO
C)LiNO2
D)(NH4)2SO4
A)RbI
B)NaCH3COO
C)LiNO2
D)(NH4)2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
24
How many milliliters of 0.653 M NaOH are needed to neutralize 25.0 mL of a 1.02 M HBr solution? The neutralization reaction is: NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
A)16.0 mL NaOH
B)25.5 mL NaOH
C)39.1 mL NaOH
D)16.3 mL NaOH
A)16.0 mL NaOH
B)25.5 mL NaOH
C)39.1 mL NaOH
D)16.3 mL NaOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH)2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH)2(aq)+ 2 HNO3(aq) 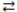 2 H2O(l)+ Ba(NO3)2(aq)
2 H2O(l)+ Ba(NO3)2(aq)
A)0.402 M HNO3
B)0.156 M HNO3
C)0.311 M HNO3
D)0.201 M HNO3
E)0.803 M HNO3
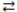 2 H2O(l)+ Ba(NO3)2(aq)
2 H2O(l)+ Ba(NO3)2(aq)A)0.402 M HNO3
B)0.156 M HNO3
C)0.311 M HNO3
D)0.201 M HNO3
E)0.803 M HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which solution has the lowest pH?
A)1.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M -OH
D)3.9 × 10-8 M -OH
E)2.3 × 10-3 M -OH
A)1.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M -OH
D)3.9 × 10-8 M -OH
E)2.3 × 10-3 M -OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the conjugate base of the hydronium ion, H3O+?
A)OH-
B)H2O-
C)H2O
D)H3O+ has no conjugate base
A)OH-
B)H2O-
C)H2O
D)H3O+ has no conjugate base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
28
What are the products of the acid-base reaction of sodium carbonate with acetic acid?
A)CO2(g)+ H2O(l)+ 2 NaCH3COO(aq)
B)5 CO2(g)+ 2 H2O(l)+ 2 NaOH(aq)
C)H2CO3(aq)+ H2O(l)+ 2 NaOH(aq)
D)CO(g)+ H2O(l)+ 2 NaCH3COO(aq)
A)CO2(g)+ H2O(l)+ 2 NaCH3COO(aq)
B)5 CO2(g)+ 2 H2O(l)+ 2 NaOH(aq)
C)H2CO3(aq)+ H2O(l)+ 2 NaOH(aq)
D)CO(g)+ H2O(l)+ 2 NaCH3COO(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the pH of a peach with a [-OH] = 3.2 × 10-11 M -OH?
A)11.32
B)10.49
C)3.51
D)3.2
A)11.32
B)10.49
C)3.51
D)3.2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
30
The pH of a lime is 1.90. What is the [H3O+]?
A)1.3 × 10-2 M H3O+
B)1.3 × 1012 M H3O+
C)7.9 × 101 M H3O+
D)7.9 × 10-13 M H3O+
E)1.9 M H3O+
A)1.3 × 10-2 M H3O+
B)1.3 × 1012 M H3O+
C)7.9 × 101 M H3O+
D)7.9 × 10-13 M H3O+
E)1.9 M H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
31
The pH of normal blood is 7.4. The pH of a diabetic's blood was determined to be 6.4. Which comparison of these two blood samples is accurate?
A)The diabetic's blood has a lower [H3O+] than normal blood.
B)The diabetic's blood is more acidic than normal blood.
C)Normal blood has a lower [OH-] than the diabetic's blood.
D)Normal blood has a higher [H3O+] than the diabetic's blood.
A)The diabetic's blood has a lower [H3O+] than normal blood.
B)The diabetic's blood is more acidic than normal blood.
C)Normal blood has a lower [OH-] than the diabetic's blood.
D)Normal blood has a higher [H3O+] than the diabetic's blood.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the pH of a buffer that contains 0.15 M CH3COOH and 0.10 M NaCH3COO (Ka = 1.8 × 10-5)?
A)4.74
B)7.00
C)4.57
D)4.92
A)4.74
B)7.00
C)4.57
D)4.92

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which term correctly describes the medical condition in which the pH of blood is greater than 7.45, and therefore the blood is more basic than normal?
A)respiratory alkalosis
B)alkalosis
C)respiratory acidosis
D)acidosis
A)respiratory alkalosis
B)alkalosis
C)respiratory acidosis
D)acidosis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
34
An aqueous solution with a low pH is necessary for a certain industrial process. Which of the following solutions would have the lowest pH?
A)2.0 M NaOH
B)0.5 M HCl
C)1.0 M acetic acid, CH3COOH
D)1.0 M HCl
E)0.15 M NaOH
A)2.0 M NaOH
B)0.5 M HCl
C)1.0 M acetic acid, CH3COOH
D)1.0 M HCl
E)0.15 M NaOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the net ionic equation for the acid-base reaction of hydrobromic acid with sodium hydroxide?
A)NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
B)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H2O(l)+ Na+(aq)+ Br-(aq)
C)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H+(aq)+ -OH(aq)+ Na+(aq)+ Br-(aq)
D)-OH(aq)+ H+(aq) H2O(l)
A)NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
B)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H2O(l)+ Na+(aq)+ Br-(aq)
C)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H+(aq)+ -OH(aq)+ Na+(aq)+ Br-(aq)
D)-OH(aq)+ H+(aq) H2O(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which solution containing an equal number of moles of each of the substances is a buffer?
A)HCl and NaCl
B)HNO2 and HNO3
C)CH3COOH and NaCH3COO
D)H2CO3 and CO32-
E)More than one of the solutions above is a buffer.
A)HCl and NaCl
B)HNO2 and HNO3
C)CH3COOH and NaCH3COO
D)H2CO3 and CO32-
E)More than one of the solutions above is a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the molarity of an HCl solution if 43.6 mL of a 0.125 M KOH solution are needed to titrate a 25.0 mL sample of the acid according to the equation below? KOH(aq)+ HCl(aq) H2O(l)+ KCl(aq)
A)0.218 M HCl
B)0.573 M HCl
C)0.0717 M HCl
D)4.58 M HCl
E)1.74 M HCl
A)0.218 M HCl
B)0.573 M HCl
C)0.0717 M HCl
D)4.58 M HCl
E)1.74 M HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which diagram properly depicts the dissolved species present in an aqueous solution of KOH? (water molecules are not shown)
A)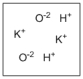
B)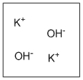
C)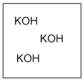
D)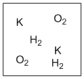
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which salt forms a basic solution when dissolved in water?
A)KCl
B)NH4Br
C)LiNO3
D)Na3PO4
A)KCl
B)NH4Br
C)LiNO3
D)Na3PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the balanced equation for the acid-base reaction of nitrous acid with lithium hydroxide?
A)LiOH(aq)+ HNO3(aq)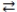 H2O(l)+ LiNO3(aq)
H2O(l)+ LiNO3(aq)
B)Li(OH)2(aq)+ 2 HNO3(aq)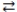 2 H2O(l)+ Li(NO3)2(aq)
2 H2O(l)+ Li(NO3)2(aq)
C)LiOH(aq)+ HNO2(aq)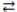 H2O(l)+ LiNO2(aq)
H2O(l)+ LiNO2(aq)
D)2 LiOH(aq)+ H2NO2(aq)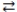 2 H2O(l)+ Li2NO2(aq)
2 H2O(l)+ Li2NO2(aq)
A)LiOH(aq)+ HNO3(aq)
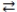 H2O(l)+ LiNO3(aq)
H2O(l)+ LiNO3(aq)B)Li(OH)2(aq)+ 2 HNO3(aq)
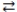 2 H2O(l)+ Li(NO3)2(aq)
2 H2O(l)+ Li(NO3)2(aq)C)LiOH(aq)+ HNO2(aq)
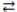 H2O(l)+ LiNO2(aq)
H2O(l)+ LiNO2(aq)D)2 LiOH(aq)+ H2NO2(aq)
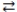 2 H2O(l)+ Li2NO2(aq)
2 H2O(l)+ Li2NO2(aq)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
41
All compounds can be classified as either an acid or a base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
42
The pH of a 1.0 × 10-5 M H3O+ solution is 5.00.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
43
A solution in which [-OH] = 6.3 × 10-7 M has a higher pH than a solution with a [-OH] = 4.3 × 10-2 M.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
44
An aqueous solution of NaHCO3 is basic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
45
A sample of water from the Chesapeake Bay has [H3O+]=3.1 × 10-9 M. Which statement below accurately describes this water sample?
A)The pH of the water sample is 7.5.
B)The water sample is a basic solution.
C)The water sample doesn't contain any hydroxide ions.
D)The water sample has a higher concentration of hydronium ions than pure water does.
A)The pH of the water sample is 7.5.
B)The water sample is a basic solution.
C)The water sample doesn't contain any hydroxide ions.
D)The water sample has a higher concentration of hydronium ions than pure water does.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
46
When phosphoric acid (H3PO4)dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O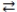 H3O+ + H2PO4-
H3O+ + H2PO4-
A)An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B)An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C)An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D)An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.
H3PO4 + H2O
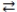 H3O+ + H2PO4-
H3O+ + H2PO4-A)An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B)An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C)An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D)An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
47
If two solutions differ in their [H3O+] by a factor of 2.0, the difference in their pH will be 2.0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
48
The pH of a 1.0 × 10-6 M -OH solution is 6.00.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
49
The value of Kw = 1.0 × 10-14 at all temperatures below 100 °C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
50
An aqueous solution of KCl has a lower pH than an aqueous solution of Li2SO3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
51
A solution in which [H3O+] = 7.4 × 10-5 M has a higher pH than a solution with a [-OH] = 4.8 × 10-8 M.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
52
The products are favored in the acid-base reaction: HI(aq)+ NH3(aq)  NH4+(aq)+ I-(aq).
NH4+(aq)+ I-(aq).
 NH4+(aq)+ I-(aq).
NH4+(aq)+ I-(aq).
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
53
A salt derived from a strong base and a weak acid forms an acidic solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
54
In an acidic solution, [H3O+] > [-OH].

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
55
Normal gastric juice has a pH of about 2. Assuming that normal gastric juice is primarily aqueous HCl, what is the concentration of HCl in the stomach?
A)2 M HCl
B)1.0 × 102 M HCl
C)0.01 M HCl
D)0.14 M HCl
A)2 M HCl
B)1.0 × 102 M HCl
C)0.01 M HCl
D)0.14 M HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
56
Although a Brønsted-Lowry acid must contain a hydrogen atom, it may be a neutral molecule or contain a net positive or negative charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
57
A sample of urine has a pH of 8.2. Which statement describing the urine sample is NOT true?
A)The urine sample is basic.
B)The urine sample has a hydronium ion concentration greater than that of a neutral solution.
C)The urine sample has a hydroxide ion concentration greater than its hydronium ion concentration.
D)The urine sample has a hydroxide ion concentration greater than 1.0 × 10-7.
A)The urine sample is basic.
B)The urine sample has a hydronium ion concentration greater than that of a neutral solution.
C)The urine sample has a hydroxide ion concentration greater than its hydronium ion concentration.
D)The urine sample has a hydroxide ion concentration greater than 1.0 × 10-7.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
58
A compound can be an acid or a base, but not both.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
59
The acid-base indicator phenolphthalein is colorless in acidic solutions and bright pink in basic solutions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
60
The value of Kw applies to any aqueous solution at 25 °C , not just pure water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
61
The addition of a base to water decreases the pH of the solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
62
Two species that differ by the presence of a proton are called a conjugate acid-base pair.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
63
An aqueous solution whose pH is greater than 7 has a higher [H3O+] than pure water itself.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
64
The principal buffer in the blood is carbonic acid/bicarbonate (H2CO3/HCO3-), keeping the normal blood pH of a healthy individual in the range of 7.35 to 7.45.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
65
OH- and NH4+ are both examples of Brønsted-Lowry bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
66
If the pH of blood is lower than 7.35, the blood is more acidic than normal, and the condition is called alkalosis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
67
In an acid-base reaction, a proton is transferred from the acid (HA)to the base (B:).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
68
All salts where the anion is derived from a strong acid form basic solutions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
69
In the reaction: SO32-(aq)+ H2O(l) 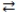 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
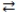 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
70
A Brønsted-Lowry base must contain a lone pair of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
71
HOBr and CH3CH2CH2COOH are both examples of Brønsted-Lowry acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
72
An aqueous solution containing an equal number of moles of Na2HPO4 and H3PO4 is an example of a buffer solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
73
Consider two acids, A and B. If acid A is weaker than acid B, then acid B dissociates in water to a greater extent than acid A.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
74
Consider two acids, A and B. If acid A is stronger than acid B, then the conjugate base of B is stronger than the conjugate base of A.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
75
An aqueous solution containing an equal number of moles of NaF and HF is an example of a buffer solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
76
For a solution labeled 0.15 M CH3COOH, the [H3O+] is 0.15 M.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
77
Water is amphoteric; it can act as an acid or a base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
78
Most acids are weak acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
79
The addition of an acid to water increases the [H3O+] of the solution and increases the solution pH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
80
A solution containing a low concentration HCl is a weak acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck








