Deck 6: Energy Changes, Reaction Rates, and Equilibrium
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/88
العب
ملء الشاشة (f)
Deck 6: Energy Changes, Reaction Rates, and Equilibrium
1
Which term correctly describes a reaction in which the energy of the products is higher than the energy of the reactants?
A)oxidation-reduction
B)endothermic
C)exothermic
D)combustion
A)oxidation-reduction
B)endothermic
C)exothermic
D)combustion
B
2
Which of the following is a true statement about the energy diagram shown below? 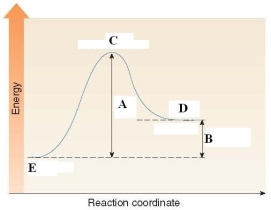
A)A labels the Ea of the reaction and B labels H of the reaction.
B)A labels the H of the reaction and B labels Ea of the reaction.
C)C labels the activation energy.
D)D labels the reactants and E labels the products.

A)A labels the Ea of the reaction and B labels H of the reaction.
B)A labels the H of the reaction and B labels Ea of the reaction.
C)C labels the activation energy.
D)D labels the reactants and E labels the products.
A labels the Ea of the reaction and B labels H of the reaction.
3
In the reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g), increasing the concentration of C2H6(g)will
A)increase the activation energy of the reaction.
B)decrease the activation energy of the reaction.
C)decrease the H of the reaction.
D)increase the reaction rate.
E)decrease the reaction rate.
A)increase the activation energy of the reaction.
B)decrease the activation energy of the reaction.
C)decrease the H of the reaction.
D)increase the reaction rate.
E)decrease the reaction rate.
increase the reaction rate.
4
An equilibrium constant with a value of 1.5 × 10-9 indicates that at equilibrium
A)the reactants are favored.
B)the products are favored.
C)approximately equal concentrations of reactants and products are present.
D)there are more products present than reactants.
A)the reactants are favored.
B)the products are favored.
C)approximately equal concentrations of reactants and products are present.
D)there are more products present than reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following energy quantities is equivalent to 11.9 kcal?
A)1.19 × 105 cal
B)49.8 J
C)49.8 kJ
D)2.84 × 103 kJ
A)1.19 × 105 cal
B)49.8 J
C)49.8 kJ
D)2.84 × 103 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
6
Consider the reaction, C2H4(g)+ H2(g) C2H6(g), where H = -137 kJ. How many kilojoules are released when 3.5 mol of C2H4 reacts?
A)137 kJ are released
B)570 kJ are released
C)480 kJ are released
D)2.0 × 103 kJ are released
A)137 kJ are released
B)570 kJ are released
C)480 kJ are released
D)2.0 × 103 kJ are released

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following energy quantities is equivalent to 258 Cal?
A)258 cal
B)1080 J
C)1080 kJ
D)6.17 × 104 kJ
A)258 cal
B)1080 J
C)1080 kJ
D)6.17 × 104 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following energy quantities is equivalent to 578 J?
A)5.78 × 105 kJ
B)138 kcal
C)0.138 kcal
D)1.38 × 105 kcal
A)5.78 × 105 kJ
B)138 kcal
C)0.138 kcal
D)1.38 × 105 kcal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
9
A peanut butter and jelly sandwich contains 9.00 g of fat, 6.00 g of protein, and 25.0 g of carbohydrate. How many Calories does this sandwich provide?
A)205 Cal
B)360 Cal
C)160 Cal
D)260 Cal
E)190 Cal
A)205 Cal
B)360 Cal
C)160 Cal
D)260 Cal
E)190 Cal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which bond is the weakest?
A)H-Br
B)H-Cl
C)H-F
D)H-I
A)H-Br
B)H-Cl
C)H-F
D)H-I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
11
Consider the reaction, C2H4(g)+ H2(g) C2H6(g), where H = -137 kJ. How many kilojoules are released when 55.3 g of C2H4 reacts?
A)137 kJ are released
B)270. kJ are released
C)1.13 × 103 kJ are released
D)7.58 × 103 kJ are released
A)137 kJ are released
B)270. kJ are released
C)1.13 × 103 kJ are released
D)7.58 × 103 kJ are released

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which bond is the strongest?
A)H-Br
B)H-Cl
C)H-F
D)H-I
A)H-Br
B)H-Cl
C)H-F
D)H-I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
13
The law of conservation of energy states that
A)the energy of the reactants and products in a chemical reaction are always equal.
B)all chemical reactions are reversible.
C)energy can be created, but not destroyed.
D)energy cannot be created or destroyed.
A)the energy of the reactants and products in a chemical reaction are always equal.
B)all chemical reactions are reversible.
C)energy can be created, but not destroyed.
D)energy cannot be created or destroyed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which quantity represents the largest amount of stored energy?
A)10 grams of fat
B)10 grams of carbohydrate
C)10 grams of protein
D)5 grams of carbohydrate plus 5 grams of protein
E)5 grams of protein plus 5 grams of fat
A)10 grams of fat
B)10 grams of carbohydrate
C)10 grams of protein
D)5 grams of carbohydrate plus 5 grams of protein
E)5 grams of protein plus 5 grams of fat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
15
An equilibrium constant with a value of 8.0 × 106 indicates that at equilibrium
A)the reactants are favored.
B)the products are favored.
C)approximately equal concentrations of reactants and products are present.
D)there are more reactants present than products.
A)the reactants are favored.
B)the products are favored.
C)approximately equal concentrations of reactants and products are present.
D)there are more reactants present than products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
16
Consider the reaction: C3H8(g)+ 5 O2(g) 3 CO2(g)+ 4 H2O(g), where H = -531 kcal. Which statement concerning this reaction is true?
A)Heat is absorbed.
B)The bonds formed in the products are stronger than the bonds broken in the reactants.
C)The products are higher in energy than the reactants.
D)The reaction is endothermic.
A)Heat is absorbed.
B)The bonds formed in the products are stronger than the bonds broken in the reactants.
C)The products are higher in energy than the reactants.
D)The reaction is endothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
17
A chemical reaction releases 55.2 kcal. How many kilojoules does this correspond to?
A)231 kJ
B)0.231 kJ
C)13.2 kJ
D)1, 320 kJ
A)231 kJ
B)0.231 kJ
C)13.2 kJ
D)1, 320 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
18
A chemical reaction requires 31.39 kJ. How many kilocalories does this correspond to?
A)7, 502 kcal
B)7.502 kcal
C)131.3 kcal
D)0.1313 kcal
E)31.39 kcal
A)7, 502 kcal
B)7.502 kcal
C)131.3 kcal
D)0.1313 kcal
E)31.39 kcal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
19
Consider the reaction, 3 NO2(g)+ H2O(l) 2 HNO3(aq)+ NO(g), where H = -137 kJ. How many kilojoules are released when 92.3 g of NO2 reacts?
A)2.01 kJ are released
B)2.75 × 102 kJ are released
C)91.6 kJ are released
D)1.26 × 104 kJ are released
A)2.01 kJ are released
B)2.75 × 102 kJ are released
C)91.6 kJ are released
D)1.26 × 104 kJ are released

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
20
Catalysts accelerate a reaction by
A)lowering the enthalpy of the reaction.
B)lowering the energy of activation.
C)raising the enthalpy of the reaction.
D)raising the energy of activation.
A)lowering the enthalpy of the reaction.
B)lowering the energy of activation.
C)raising the enthalpy of the reaction.
D)raising the energy of activation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following is NOT a reasonable assumption about the chemical reaction whose energy diagram is depicted below? 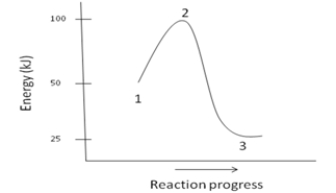
A)The activation energy for the reaction is 100kJ.
B)The reaction is exothermic.
C) H= -25kJ
D)The reaction is favorable.

A)The activation energy for the reaction is 100kJ.
B)The reaction is exothermic.
C) H= -25kJ
D)The reaction is favorable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
22
Consider the combustion reaction of propane: C3H8(g)+ 5 O2(g) 3 CO2(g)+ 4 H2O(g), where H = -531 kcal. If 6.70 × 104 kcal of energy is released in the reaction, how many grams of oxygen were consumed?
A)63.1 g of O2
B)2.02 × 104 g of O2
C)12.6 g of O2
D)404 g of O2
A)63.1 g of O2
B)2.02 × 104 g of O2
C)12.6 g of O2
D)404 g of O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
23
Consider the reversible reaction at equilibrium: N2(g)+ O2(g)  2 NO(g). What is the effect of removing some N2(g)from the equilibrium system?
2 NO(g). What is the effect of removing some N2(g)from the equilibrium system?
A)the concentration of O2(g)increases
B)the concentration of O2(g)decreases
C)the concentration of NO(g)increases
D)the equilibrium system shifts to the right
 2 NO(g). What is the effect of removing some N2(g)from the equilibrium system?
2 NO(g). What is the effect of removing some N2(g)from the equilibrium system?A)the concentration of O2(g)increases
B)the concentration of O2(g)decreases
C)the concentration of NO(g)increases
D)the equilibrium system shifts to the right

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
24
Consider the reaction: C3H8(g)+ 5 O2(g) 3 CO2(g)+ 4 H2O(g), where H = -531 kcal/mol. How much heat is released when 3.40 × 1020 molecules of C3H8(g)is burned?
A)3.33 kcal
B)0.300 kcal
C)6.40 × 1017 kcal
D)1.50 kcal
A)3.33 kcal
B)0.300 kcal
C)6.40 × 1017 kcal
D)1.50 kcal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
25
The molecular art depicts the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 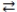 COCl2(g). What can be inferred about the equilibrium constant, K, for this reaction?
COCl2(g). What can be inferred about the equilibrium constant, K, for this reaction? 
A)K < 1
B)K ~ 1
C)K > 1
D)K = 0
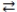 COCl2(g). What can be inferred about the equilibrium constant, K, for this reaction?
COCl2(g). What can be inferred about the equilibrium constant, K, for this reaction? 
A)K < 1
B)K ~ 1
C)K > 1
D)K = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
26
Consider the reaction: PCl3(g)+ Cl2(g) ![<strong>Consider the reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g). If [PCl<sub>3</sub>] = 0.78 M, [Cl<sub>2</sub>] = 0.44 M, and [PCl<sub>5</sub>] = 0.88 at equilibrium, what is the value of K?</strong> A)0.39 B)1.4 C)2.6 D)0.72](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e4f_9547_854b454e379f_TB5866_11.jpg) PCl5(g). If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?
PCl5(g). If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?
A)0.39
B)1.4
C)2.6
D)0.72
![<strong>Consider the reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g). If [PCl<sub>3</sub>] = 0.78 M, [Cl<sub>2</sub>] = 0.44 M, and [PCl<sub>5</sub>] = 0.88 at equilibrium, what is the value of K?</strong> A)0.39 B)1.4 C)2.6 D)0.72](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e4f_9547_854b454e379f_TB5866_11.jpg) PCl5(g). If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?
PCl5(g). If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?A)0.39
B)1.4
C)2.6
D)0.72

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
27
Consider the reaction: N2(g)+ O2(g) ![<strong>Consider the reaction: N<sub>2</sub>(g)+ O<sub>2</sub>(g) 2 NO(g). If [N<sub>2</sub>] = 0.520 M, [O<sub>2</sub>] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?</strong> A)3.60 B)0.278 C)377 D)0.00265](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e50_9547_8585aa67b6d2_TB5866_11.jpg) 2 NO(g). If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?
2 NO(g). If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?
A)3.60
B)0.278
C)377
D)0.00265
![<strong>Consider the reaction: N<sub>2</sub>(g)+ O<sub>2</sub>(g) 2 NO(g). If [N<sub>2</sub>] = 0.520 M, [O<sub>2</sub>] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?</strong> A)3.60 B)0.278 C)377 D)0.00265](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e50_9547_8585aa67b6d2_TB5866_11.jpg) 2 NO(g). If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?
2 NO(g). If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?A)3.60
B)0.278
C)377
D)0.00265

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
28
A catalytic converter uses a catalyst to catalyze three reactions that clean up the exhaust from an auto engine. Which element is not used as a catalyst in catalytic converters?
A)platinum
B)rhodium
C)sulfur
D)palladium
A)platinum
B)rhodium
C)sulfur
D)palladium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following is always necessary for a chemical reaction to occur between two reactants, A and B?
A)Equal amounts of A and B must be present
B)A and B must be present in the same physical state
C)The reaction must be carried out at a temperature higher than room temperature
D)A and B must collide with the proper orientation and with a certain minimum amount of energy
A)Equal amounts of A and B must be present
B)A and B must be present in the same physical state
C)The reaction must be carried out at a temperature higher than room temperature
D)A and B must collide with the proper orientation and with a certain minimum amount of energy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
30
Walking at a brisk pace burns off about 280 Cal/h. How long would you have to walk to burn off the Calories obtained from eating a candy bar that contained 3 g of protein, 12 g of fat, and 28 g of carbohydrates?
A)55 minutes
B)230 minutes
C)50 minutes
D)210 minutes
A)55 minutes
B)230 minutes
C)50 minutes
D)210 minutes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
31
The reversible reaction: PCl3(g)+ Cl2(g)  PCl5(g), has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions?
PCl5(g), has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions? 
A)The reaction has not yet reached equilibrium.
B)The reaction mixture is at equilibrium.
C)The reaction will shift to the left to reach equilibrium.
D)The forward reaction is favorable.
 PCl5(g), has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions?
PCl5(g), has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions? 
A)The reaction has not yet reached equilibrium.
B)The reaction mixture is at equilibrium.
C)The reaction will shift to the left to reach equilibrium.
D)The forward reaction is favorable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the gas phase chemical reaction that corresponds to the equilibrium constant expression shown below? 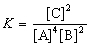
A)2 A + 4 B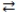 2 C
2 C
B)A + B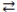 C
C
C)2 C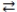 2 A + 4 B
2 A + 4 B
D)2 C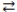 4 A + 2 B
4 A + 2 B
E)4 A + 2 B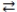 2 C
2 C
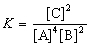
A)2 A + 4 B
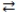 2 C
2 CB)A + B
 C
CC)2 C
 2 A + 4 B
2 A + 4 BD)2 C
 4 A + 2 B
4 A + 2 BE)4 A + 2 B
 2 C
2 C
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
33
The rusting of iron is described by the reaction: 4 Fe(s)+ 3 O2(g) 2 Fe2O3(s). The formation of rust on an exposed piece of iron typically takes several months. Which of the following is NOT a reasonable assumption about this chemical reaction?
A)The formation of rust has a low reaction rate.
B)The formation of rust has a low activation energy.
C)The formation of rust would occur more slowly at higher altitudes where the concentration of oxygen is lower.
D)The formation of rust would occur faster in the warmer summer months than in the cooler winter months.
A)The formation of rust has a low reaction rate.
B)The formation of rust has a low activation energy.
C)The formation of rust would occur more slowly at higher altitudes where the concentration of oxygen is lower.
D)The formation of rust would occur faster in the warmer summer months than in the cooler winter months.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
34
When the pressure of a reaction at equilibrium decreases, in which direction does the equilibrium shift?
A)The equilibrium shifts in the direction that increases the number of moles of gas.
B)The equilibrium shifts in the direction that decreases the number of moles of gas.
C)The equilibrium does not shift.
D)The equilibrium shifts always shifts to the right.
E)The equilibrium shifts always shifts to the left.
A)The equilibrium shifts in the direction that increases the number of moles of gas.
B)The equilibrium shifts in the direction that decreases the number of moles of gas.
C)The equilibrium does not shift.
D)The equilibrium shifts always shifts to the right.
E)The equilibrium shifts always shifts to the left.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
35
For an endothermic reaction at equilibrium, increasing the temperature
A)does not shift the equilibrium since K is a constant.
B)increases the rate of the reverse reaction to form more reactants.
C)increases the rate of the forward reaction to form more products.
D)increases the rate of the reverse reaction to form more products.
A)does not shift the equilibrium since K is a constant.
B)increases the rate of the reverse reaction to form more reactants.
C)increases the rate of the forward reaction to form more products.
D)increases the rate of the reverse reaction to form more products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following will increase the rate of a reaction?
A)increasing the temperature
B)increasing the concentration of a reactant
C)adding a catalyst
D)ensuring that the reactants are properly aligned when they collide
E)All of the above changes will increase the rate of a reaction.
A)increasing the temperature
B)increasing the concentration of a reactant
C)adding a catalyst
D)ensuring that the reactants are properly aligned when they collide
E)All of the above changes will increase the rate of a reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
37
The rate of a chemical reaction increases with an increase in the concentration of one or more reactants. This is best explained by which statement?
A)The higher concentration of reactants increases the potential energy of the molecules.
B)The higher concentration of reactants increases the activation energy of the reaction.
C)The higher concentration of reactants increases the temperature of the molecules.
D)The higher concentration of reactants increases the frequency of collisions between molecules.
A)The higher concentration of reactants increases the potential energy of the molecules.
B)The higher concentration of reactants increases the activation energy of the reaction.
C)The higher concentration of reactants increases the temperature of the molecules.
D)The higher concentration of reactants increases the frequency of collisions between molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which statement about catalysts is NOT true?
A)A catalyst increases the rate of a chemical reaction.
B)A catalyst lowers the activation energy of a chemical reaction.
C)A catalyst lowers the H of a chemical reaction.
D)A catalyst is recovered unchanged in the reaction it catalyzes.
A)A catalyst increases the rate of a chemical reaction.
B)A catalyst lowers the activation energy of a chemical reaction.
C)A catalyst lowers the H of a chemical reaction.
D)A catalyst is recovered unchanged in the reaction it catalyzes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
39
A reversible reaction has reached equilibrium when which condition is established?
A)the reverse reaction begins to occur
B)the concentrations of reactants and products become equal
C)all of the reactants have been converted into products
D)the forward and reverse reaction rates become equal
A)the reverse reaction begins to occur
B)the concentrations of reactants and products become equal
C)all of the reactants have been converted into products
D)the forward and reverse reaction rates become equal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
40
Consider the combustion reaction of propane: C3H8(g)+ 5 O2(g) 3 CO2(g)+ 4 H2O(g), where H = -531 kcal. If 1.24 × 105 kcal of energy is released in the reaction, how many moles of propane were burned?
A)234 mol of C3H8
B)6.58 × 107 mol of C3H8
C)0.00428 mol of C3H8
D)0.0179 mol of C3H8
A)234 mol of C3H8
B)6.58 × 107 mol of C3H8
C)0.00428 mol of C3H8
D)0.0179 mol of C3H8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
41
In the energy diagram shown below, the H of the reaction is labeled by D. 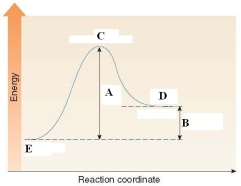


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
42
The stronger the bond, the higher its bond dissociation energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
43
Increasing the concentration of the reactants in a chemical reaction increases the number of collisions, and the reaction rate increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
44
Ammonia (NH3)is synthesized by the reaction of nitrogen and hydrogen in the presence of an iron catalyst according to the equation below. Removing the iron catalyst would decrease the reaction rate. 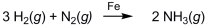
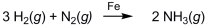

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which statement concerning the reversible reaction 2 NO2(g)  N2O4(g)is true?
N2O4(g)is true?
A)NO2 is the product of the forward reaction.
B)The reverse reaction produces N2O4.
C)At the start of the reaction, the forward and reverse reaction rates are equal.
D)As the forward reaction progresses and more N2O4 is formed, the reverse reaction rate increases.
 N2O4(g)is true?
N2O4(g)is true?A)NO2 is the product of the forward reaction.
B)The reverse reaction produces N2O4.
C)At the start of the reaction, the forward and reverse reaction rates are equal.
D)As the forward reaction progresses and more N2O4 is formed, the reverse reaction rate increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
46
Exothermic reactions involve the formation of products having lower energy than the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
47
Activation energy is the minimum amount of energy necessary for a reaction to occur.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
48
The difference in energy between the reactants and the transition state is called the energy of activation, symbolized by Ea.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
49
Breaking a chemical bond requires energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
50
The hydrolysis of sucrose depicted below has K = 1.4 × 105. Which of the following is a reasonable assumption about this reaction once equilibrium is established? sucrose + H2O  glucose + fructose
glucose + fructose
A)The equilibrium mixture contains equal amounts of sucrose, glucose, and fructose.
B)The equilibrium mixture contains mostly glucose and fructose.
C)The equilibrium mixture contains mostly sucrose.
D)The equilibrium shifts to the left due to the high value for K.
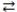 glucose + fructose
glucose + fructoseA)The equilibrium mixture contains equal amounts of sucrose, glucose, and fructose.
B)The equilibrium mixture contains mostly glucose and fructose.
C)The equilibrium mixture contains mostly sucrose.
D)The equilibrium shifts to the left due to the high value for K.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
51
Chemical reactions are considered favorable if the products have a higher energy than the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
52
Changes in potential energy occur in chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
53
One step in the metabolism of glucose is depicted below. Which statement best describes how the equilibrium system would respond if the amount of dihydroxyacetone phosphate is decreased? 
A)The system would shift to the left, consuming more dihydroxyacetone phosphate.
B)The system would shift to the right, producing more dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
C)The system would shift to the left, producing more fructose 1, 6-bisphosphate.
D)The system would shift to the right, consuming some glyceraldehyde 3-phosphate and producing more dihydroxyacetone phosphate.

A)The system would shift to the left, consuming more dihydroxyacetone phosphate.
B)The system would shift to the right, producing more dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
C)The system would shift to the left, producing more fructose 1, 6-bisphosphate.
D)The system would shift to the right, consuming some glyceraldehyde 3-phosphate and producing more dihydroxyacetone phosphate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
54
The energy of the reacting molecules affects whether a particular collision will lead to a chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
55
Increasing the temperature of a reaction mixture usually results in a decrease in the reaction rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
56
Reactions with high Ea are generally fast reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
57
Energy is the capacity to do work.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
58
An endothermic reaction is one in which energy is absorbed and H is negative (-).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
59
Bond breaking is endothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
60
Bond dissociation energies are always positive numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
61
The expression for the equilibrium constant, K, for the general reaction: a A + b B 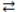 c C + d D is
c C + d D is 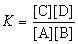 .
.
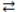 c C + d D is
c C + d D is 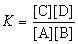 .
.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
62
Once equilibrium is reached in a chemical reaction, reactants stop forming products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
63
Consider the reversible reaction: PCl3(g)+ Cl2(g) ![Consider the reversible reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g), where K = 0.5. Since K < 1, [PCl<sub>5</sub>] must be less than 1 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de43_94ff_9547_3144d1bb3758_TB5866_11.jpg) PCl5(g), where K = 0.5. Since K < 1, [PCl5] must be less than 1 M.
PCl5(g), where K = 0.5. Since K < 1, [PCl5] must be less than 1 M.
![Consider the reversible reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g), where K = 0.5. Since K < 1, [PCl<sub>5</sub>] must be less than 1 M.](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de43_94ff_9547_3144d1bb3758_TB5866_11.jpg) PCl5(g), where K = 0.5. Since K < 1, [PCl5] must be less than 1 M.
PCl5(g), where K = 0.5. Since K < 1, [PCl5] must be less than 1 M.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
64
When the equilibrium constant for a reaction is much less than one (K < 1), the concentration of the products is larger than the concentration of the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
65
Consider the reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g). Increasing the concentration of C2H6(g)will decrease the activation energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
66
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 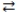 COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
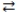 COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
67
A manufacturing company requires 157 kJ of energy to power one of its machines for a day. The reaction shown below would be able to provide a sufficient amount of energy for this purpose.
2 HgO(s) 2 Hg(l)+ O2(g) H = 182 kJ
2 HgO(s) 2 Hg(l)+ O2(g) H = 182 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
68
A reversible reaction is said to have reached equilibrium when the concentrations of the reactants and products become equal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
69
The larger the K for a reaction, the faster the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
70
Consider the reversible reaction: CO(g)+ Cl2(g) 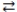 COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g).
 COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g). 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
71
When the equilibrium constant for a reversible reaction is much greater than one (K > 1), the equilibrium is said to lie to the right.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
72
When H is negative, the bonds formed in the products are _____ than the bonds broken in the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
73
Kinetic energy is the energy associated with movement; potential energy is the energy inherent in an object due to its position or composition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
74
A reversible reaction in which K = 9.65 × 10-14 contains a negligible amount of reactants at equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
75
Consider the reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g). Decreasing the concentration of C2H6(g)will decrease the reaction rate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
76
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g)  COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
 COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
77
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g)  COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
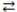 COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2. 
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
78
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 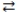 COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
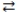 COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
79
Enzymes are proteins that act as biological catalysts.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
80
Le Châtelier's principle is a general rule used to explain the effect of a change in reaction conditions on equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck








