Deck 4: Covalent Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/90
العب
ملء الشاشة (f)
Deck 4: Covalent Compounds
1
Predict the bond angles around the carbon atom in the structure of carbonic acid shown below. Don't forget to draw in lone pairs where needed to give octets. 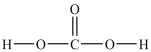
A)180°
B)120°
C)109.5°
D)90°
E)60°

A)180°
B)120°
C)109.5°
D)90°
E)60°
B
2
How many lone pairs of electrons are present in the Lewis structure of ammonia, NH3?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4
B
3
How many valence electrons are in a molecule of formaldehyde (CH2O)?
A)16
B)12
C)10
D)8
E)0
A)16
B)12
C)10
D)8
E)0
B
4
A diatomic molecule contains I. atoms of two different elements bonded together with a covalent bond.
II. two atoms of the same element bonded together with a covalent bond.
III. two lone pairs of electrons.
A)I only
B)II only
C)III only
D)I or II only
E)I and III only
II. two atoms of the same element bonded together with a covalent bond.
III. two lone pairs of electrons.
A)I only
B)II only
C)III only
D)I or II only
E)I and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
5
Rank the atoms Br, Cl, and F in order of increasing electronegativity.
A)F < Br < Cl
B)Cl < Br < F
C)Br < Cl < F
D)F < Cl < Br
E)Cl < F < Br
A)F < Br < Cl
B)Cl < Br < F
C)Br < Cl < F
D)F < Cl < Br
E)Cl < F < Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which bond is the most polar?
A)C-N
B)C-O
C)C-C
D)C-Cl
E)C-F
A)C-N
B)C-O
C)C-C
D)C-Cl
E)C-F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
7
What are the bond angles in a tetrahedral geometry?
A)180°
B)120°
C)109.5°
D)90°
E)60°
A)180°
B)120°
C)109.5°
D)90°
E)60°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
8
How many nonbonded electron pairs are in the Lewis structure below? 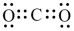
A)2
B)4
C)6
D)8
E)16
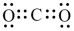
A)2
B)4
C)6
D)8
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
9
Aspartic acid is an amino acid used to synthesize proteins. How many polar bonds are in the aspartic acid structure shown below? 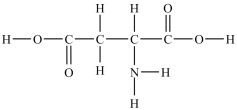
A)4
B)6
C)9
D)12
E)15

A)4
B)6
C)9
D)12
E)15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the statements concerning chemical bonds is false?
A)The sharing of electrons between two nonmetal atoms results in a covalent bond.
B)The attraction between oppositely charged ions results in an ionic bond.
C)The term nonpolar is used to describe a covalent bond in which electrons are not shared equally.
D)A bond dipole is the separation of charge that results when atoms sharing electrons have different electronegativities.
A)The sharing of electrons between two nonmetal atoms results in a covalent bond.
B)The attraction between oppositely charged ions results in an ionic bond.
C)The term nonpolar is used to describe a covalent bond in which electrons are not shared equally.
D)A bond dipole is the separation of charge that results when atoms sharing electrons have different electronegativities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many covalent bonds are generally formed by atoms with five valence electrons?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
12
Rank the atoms Br, Cl, and K in order of increasing electronegativity.
A)K < Br < Cl
B)Cl < Br < K
C)Br < Cl < K
D)K < Cl < Br
E)Cl < K < Br
A)K < Br < Cl
B)Cl < Br < K
C)Br < Cl < K
D)K < Cl < Br
E)Cl < K < Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the molecular shape around the phosphorus atom in PH3?
A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal
A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the Lewis structure for chloroethylene (C2H3Cl)?
A)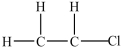
B)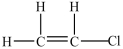
C)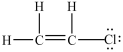
D)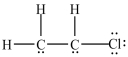
A)
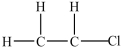
B)
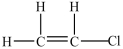
C)
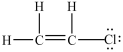
D)
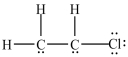

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which atom has the lowest electronegativity?
A)Al
B)S
C)Se
D)Rb
E)F
A)Al
B)S
C)Se
D)Rb
E)F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which bond is the least polar?
A)C-N
B)C-O
C)C-C
D)C-Cl
E)C-F
A)C-N
B)C-O
C)C-C
D)C-Cl
E)C-F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following molecule(s)is(are)polar?
A)CO2
B)CH4
C)CBr4
D)CHBr3
E)More than one of the above molecules are polar.
A)CO2
B)CH4
C)CBr4
D)CHBr3
E)More than one of the above molecules are polar.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
18
Covalent bonds result from the _____ electrons between two atoms.
A)transfer of
B)equal sharing of
C)unequal sharing of
D)A, B, and C are correct
E)B and C are correct
A)transfer of
B)equal sharing of
C)unequal sharing of
D)A, B, and C are correct
E)B and C are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
19
Aspartic acid is an amino acid used to synthesize proteins. How many lone pairs of electrons need to be added to complete the aspartic acid structure shown below? 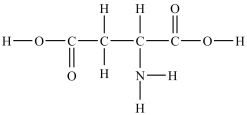
A)4
B)6
C)8
D)9
E)11

A)4
B)6
C)8
D)9
E)11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is another name for an unshared pair of electrons in a covalent compound?
A)lone pair of electrons
B)nonbonded electron pair
C)bonding pair of electrons
D)lone pair of electrons or nonbonded electron pair
A)lone pair of electrons
B)nonbonded electron pair
C)bonding pair of electrons
D)lone pair of electrons or nonbonded electron pair

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which is the correct Lewis structure for OBr-?
A)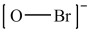
B)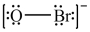
C)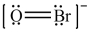
D)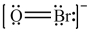
E)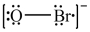
A)
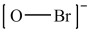
B)
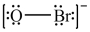
C)
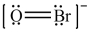
D)
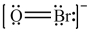
E)
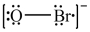

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which statement concerning chemical bonds is false?
A)A covalent bond involves the sharing of electrons between two nonmetal atoms.
B)An ionic bond is the attraction between oppositely charged ions.
C)A nonpolar bond is a covalent bond in which electrons are not shared equally between the atoms.
D)Electronegativity is a measure of the attraction an atom has for the electrons it shares in a bond.
A)A covalent bond involves the sharing of electrons between two nonmetal atoms.
B)An ionic bond is the attraction between oppositely charged ions.
C)A nonpolar bond is a covalent bond in which electrons are not shared equally between the atoms.
D)Electronegativity is a measure of the attraction an atom has for the electrons it shares in a bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the chemical formula for dinitrogen tetroxide?
A)N4O2
B)O4N2
C)N2O4
D)O2N4
E)NO2
A)N4O2
B)O4N2
C)N2O4
D)O2N4
E)NO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
24
Considering the electronegativity values indicated for each element, which covalent bond has the least degree of polarity? 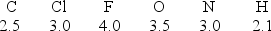
A)C-N
B)N-O
C)F-F
D)H-Cl
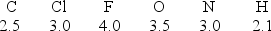
A)C-N
B)N-O
C)F-F
D)H-Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
25
Estimate the bond angles around the sulfur atom in the structure shown below. 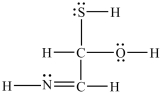
A)90°
B)109.5°
C)120°
D)180°

A)90°
B)109.5°
C)120°
D)180°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the statements concerning compounds is incorrect?
A)Compounds are formed when atoms of two or more different elements are chemically bonded.
B)Ionic compounds are composed of cations and anions.
C)Covalent compounds are composed of metals and nonmetals.
D)Covalent compounds are composed of individual molecules.
A)Compounds are formed when atoms of two or more different elements are chemically bonded.
B)Ionic compounds are composed of cations and anions.
C)Covalent compounds are composed of metals and nonmetals.
D)Covalent compounds are composed of individual molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following is classified as a group in the valence shell electron pair repulsion (VSEPR)theory?
A)an atom
B)a lone pair of electrons
C)a valence electron
D)either an atom or a valence electron
E)either an atom or a lone pair of electrons
A)an atom
B)a lone pair of electrons
C)a valence electron
D)either an atom or a valence electron
E)either an atom or a lone pair of electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which bond has the polarity incorrectly labeled?
A) + H-Cl -
B) + O-C -
C) 1 + Cl- -
D) - Cl-Br +
E) - F-O +
A) + H-Cl -
B) + O-C -
C) 1 + Cl- -
D) - Cl-Br +
E) - F-O +

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the total number of bonding electrons in the structure below? 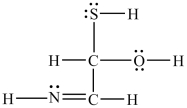
A)5
B)10
C)20
D)30

A)5
B)10
C)20
D)30

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which atom(s)in the structure below has(have)a partial negative charge ( -)? 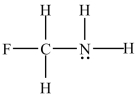
A)carbon
B)fluorine
C)hydrogen
D)nitrogen
E)nitrogen and fluorine

A)carbon
B)fluorine
C)hydrogen
D)nitrogen
E)nitrogen and fluorine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which molecule's Lewis structure contains an atom that violates the octet rule?
A)H2O
B)BeH2
C)PCl3
D)H2Se
A)H2O
B)BeH2
C)PCl3
D)H2Se

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the molecular shape around the oxygen atom in the structure shown below? 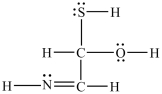
A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal

A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the molecular shape around the nitrogen atom in the structure shown below? 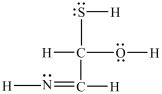
A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal

A)linear
B)bent
C)trigonal planar
D)tetrahedral
E)trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which element may have more than eight valence electrons around it when present in a covalent compound?
A)C
B)B
C)N
D)P
E)O
A)C
B)B
C)N
D)P
E)O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many lone pairs of electrons need to be added to the Lewis structure of carbonic acid shown below? 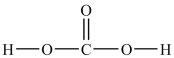
A)0
B)3
C)4
D)6
E)7

A)0
B)3
C)4
D)6
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the correct chemical formula of selenium dioxide?
A)SO2
B)SeO2
C)Se2O
D)OS2
A)SO2
B)SeO2
C)Se2O
D)OS2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which Lewis structure is incorrect?
A)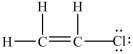
B)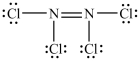
C)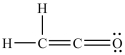
D)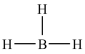
A)
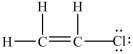
B)
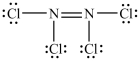
C)
D)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which molecule or ion has only two resonance structures to describe its bonding?
A)CO32-
B)NO3-
C)HCO31-
D)SO3
A)CO32-
B)NO3-
C)HCO31-
D)SO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which compound has the greatest number of valence electrons?
A)H2S
B)CH4
C)NH3
D)H2O
E)All of the molecules above have the same number of valence electrons.
A)H2S
B)CH4
C)NH3
D)H2O
E)All of the molecules above have the same number of valence electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
40
The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 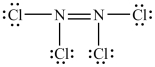
A)The nitrogen atoms violate the octet rule.
B)The chlorine atoms violate the octet rule.
C)The structure contains an incorrect number of valence electrons.
D)Chlorine atoms and nitrogen atoms do not typically form bonds with each other.
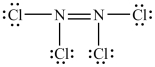
A)The nitrogen atoms violate the octet rule.
B)The chlorine atoms violate the octet rule.
C)The structure contains an incorrect number of valence electrons.
D)Chlorine atoms and nitrogen atoms do not typically form bonds with each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
41
Bonding is the joining of two atoms in a stable arrangement.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
42
A molecule is a discrete group of atoms held together by covalent bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
43
Resonance structures for a substance differ only in the location of multiple bonds and the position of lone electron pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
44
Phosphorus usually forms two covalent bonds in a molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
45
Nonpolar molecules may contain polar bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
46
Unequal sharing of electrons in a covalent bond results in a polar bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
47
The covalent bond between chlorine and iodine is a polar one. Which of the following properly represents the direction of polarity in this bond?
A) + Cl-I -
B) - Cl-I +
C)+ Cl-I -
D)- Cl-I +
A) + Cl-I -
B) - Cl-I +
C)+ Cl-I -
D)- Cl-I +

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
48
Atoms with seven valence electrons typically form one covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
49
The Lewis structure for BH3 contains an atom that does not follow the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
50
Some covalent compounds are solids, some are liquids, and some are gases at room temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
51
The Lewis structure for PH3 contains an atom that does not follow the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
52
A Lewis structure shows the connectivity between atoms in a molecule, as well as where all the bonding and nonbonding valence electrons reside.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
53
A double bond consists of four electrons shared between two atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
54
The formula for dinitrogen pentoxide is N5O2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
55
The correct name for SF6 is sulfur heptafluoride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
56
There can be a no more than two resonance structures for a molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
57
Every atom must have an octet of electrons in order for a Lewis structure to be considered valid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
58
The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is incorrect? 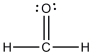
A)Two electrons are being shared between the carbon atom and the oxygen atom.
B)The oxygen atom has four valence electrons that are not being shared with another atom.
C)The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D)The hydrogen atoms have filled valence shells.

A)Two electrons are being shared between the carbon atom and the oxygen atom.
B)The oxygen atom has four valence electrons that are not being shared with another atom.
C)The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D)The hydrogen atoms have filled valence shells.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
59
Atoms with three valence electrons generally form five bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
60
A bond formed between the elements hydrogen and bromine would be considered an ionic bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
61
A double bond is counted as two groups in the valence shell electron pair repulsion (VSEPR)theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
62
A molecule that contains only one polar bond is a polar molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
63
Dibromomethane (CH2Br2)is a nonpolar molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
64
The Lewis structure for the molecule below is incomplete because it is missing six pairs of nonbonding electrons. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
65
The symbol - is given to the more electronegative atom in a polar bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
66
The structures shown below are resonance structures of sulfur dioxide. 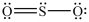 and
and 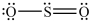
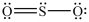 and
and 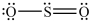

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
67
The shapes around the left and right carbon atoms in the structure below are tetrahedral and linear, respectively. 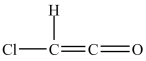
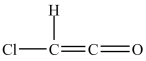

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
68
The shape around each carbon atom in the molecule below is trigonal planar. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
69
The molecular shape around the boron atom in BCl3 is different than the molecular shape around the nitrogen atom in NCl3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
70
The structures shown below are resonance structures of sulfur dioxide. 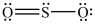 and
and 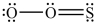
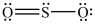 and
and 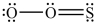

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
71
A N-O bond is more polar than a P-O bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
72
In the valence shell electron pair repulsion (VSEPR)theory, a group is defined as an atom or a lone pair of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
73
When writing Lewis structures, the symbol below is placed between resonance structures. 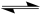

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
74
A resonance hybrid is a composite of all resonance structures that spreads out electron pairs in multiple bonds and lone pairs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
75
The molecule below is a polar molecule. 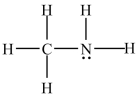


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
76
Ethane (C2H6)is a polar molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
77
A bond between a carbon atom and a nitrogen atom is a polar covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
78
Double bonds and triple bonds are never polar bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
79
C-H bonds are considered to be nonpolar, because the electronegativity difference between carbon and hydrogen is small.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck
80
The molecule below is a polar molecule. 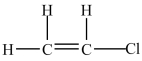
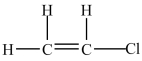

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 90 في هذه المجموعة.
فتح الحزمة
k this deck








