Deck 9: Energy, Enthalpy, and Thermochemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/81
العب
ملء الشاشة (f)
Deck 9: Energy, Enthalpy, and Thermochemistry
1
The H value for the reaction (1/2)O2(g) + Cu(s) CuO(s) is -156 kJ. How much heat is released when 31.4 g of Cu is reacted with oxygen?
A) 61.6 kJ
B) 156 kJ
C) 315 kJ
D) 77.1 kJ
E) 4.90 *103 kJ
A) 61.6 kJ
B) 156 kJ
C) 315 kJ
D) 77.1 kJ
E) 4.90 *103 kJ
77.1 kJ
2
Calculate the work for the expansion of an ideal gas from 3.1 to 6.4 L against a pressure of 1.6 atm at constant temperature.
A) 0
B) -2.1 L•atm
C) 5.3 L•atm
D) 5.9 L•atm
E) -5.3 L•atm
A) 0
B) -2.1 L•atm
C) 5.3 L•atm
D) 5.9 L•atm
E) -5.3 L•atm
-5.3 L•atm
3
Consider the reaction C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l), H = -1.37 * 103 kJ
When a 15.4-g sample of ethyl alcohol (molar mass = 46.1 g/mol) is burned, how much energy is released as heat?
A) 89.0 kJ
B) 4.58 * 102 kJ
C) 4.10 * 103 kJ
D) 4.69 kJ
E) 2.11 *104 kJ
When a 15.4-g sample of ethyl alcohol (molar mass = 46.1 g/mol) is burned, how much energy is released as heat?
A) 89.0 kJ
B) 4.58 * 102 kJ
C) 4.10 * 103 kJ
D) 4.69 kJ
E) 2.11 *104 kJ
4.58 * 102 kJ
4
The total volume of hydrogen gas needed to fill the Hindenburg was 2.00*108 L at 1.00 atm and 25.0°C. How much energy was evolved when it burned? H2(g) + (1/2)O2(g) H2O(l), H = -286 kJ
A) 8.18 *106 kJ
B) 3.5 * 1011 kJ
C) 2.86 * 104 kJ
D) 5.72 * 1010 kJ
E) 2.34 * 109 kJ
A) 8.18 *106 kJ
B) 3.5 * 1011 kJ
C) 2.86 * 104 kJ
D) 5.72 * 1010 kJ
E) 2.34 * 109 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
5
One mole of an ideal gas is expanded from a volume of 3.00 L to a volume of 10.38 L against a constant external pressure of 1.08 atm. Calculate the work. (1 L•atm = 101.3 J)
A) -7.97 J
B) -0.0787 J
C) 8.07 J
D) -7.48 * 102 J
E) -8.07 * 102 J
A) -7.97 J
B) -0.0787 J
C) 8.07 J
D) -7.48 * 102 J
E) -8.07 * 102 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
6
C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l), H = -1.37 * 103 kJ For the combustion of ethyl alcohol as described in the above equation, which of the following statements is(are) true?
I. The reaction is exothermic.
II. The enthalpy change would be different if gaseous water were produced.
III. The reaction is not an oxidation-reduction one.
IV. The products of the reaction occupy a larger volume than the reactants.
A) I, II
B) I, III, IV
C) I only
D) III, IV
E) I, II, III
I. The reaction is exothermic.
II. The enthalpy change would be different if gaseous water were produced.
III. The reaction is not an oxidation-reduction one.
IV. The products of the reaction occupy a larger volume than the reactants.
A) I, II
B) I, III, IV
C) I only
D) III, IV
E) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
7
CH4 + 4Cl2(g) CCl4(g) + 4HCl(g), H = -434 kJ Based on the above reaction, what energy change occurs when 1.8 mol of methane reacts?
A) 2.4 *105 J is released.
B) 7.8 * 105 J is released.
C) 2.4 *105 J is absorbed.
D) 7.8 *105 J is absorbed.
E) 4.3*102 J is released.
A) 2.4 *105 J is released.
B) 7.8 * 105 J is released.
C) 2.4 *105 J is absorbed.
D) 7.8 *105 J is absorbed.
E) 4.3*102 J is released.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
8
Calculate E for a system that releases 32 J of heat while 56 J of work is done on it.
A) 32 J
B) 88 J
C) 24 J
D) -88 J
E) -24 J
A) 32 J
B) 88 J
C) 24 J
D) -88 J
E) -24 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
9
A gas absorbs 2.0 J of heat and then performs 11.6 J of work. What is the change in internal energy of the gas?
A) -9.6 J
B) 9.6 J
C) -13.6 J
D) 2.0 J
E) 13.6 J
A) -9.6 J
B) 9.6 J
C) -13.6 J
D) 2.0 J
E) 13.6 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following statements is correct?
A) The system does work on the surroundings when an ideal gas expands against a constant external pressure.
B) The internal energy of a system increases when more work is done by the system than heat is flowing into the system.
C) The internal energy of a system decreases when work is done on the system and heat is flowing into the system.
D) All the statements are true.
E) All the statements are false.
A) The system does work on the surroundings when an ideal gas expands against a constant external pressure.
B) The internal energy of a system increases when more work is done by the system than heat is flowing into the system.
C) The internal energy of a system decreases when work is done on the system and heat is flowing into the system.
D) All the statements are true.
E) All the statements are false.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following are state functions?
A) work, heat, enthalpy
B) heat, enthalpy, energy
C) enthalpy, energy
D) work, heat, enthalpy, energy
E) work, heat
A) work, heat, enthalpy
B) heat, enthalpy, energy
C) enthalpy, energy
D) work, heat, enthalpy, energy
E) work, heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
12
Suppose you add 45 J of heat to a system, let it do 10. J of expansion work, and then return the system to its initial state by cooling and compression. Which statement is true for this process?
A) ( H < E)
B) ( H = 70. J)
C) The work done in compressing the system must exactly equal the work done by the system in the expansion step.
D) The change in the internal energy for this process is zero.
A) ( H < E)
B) ( H = 70. J)
C) The work done in compressing the system must exactly equal the work done by the system in the expansion step.
D) The change in the internal energy for this process is zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
13
For a particular process q = -10 kJ and w = 25 kJ. Which of the following statements is true?
A) The system does work on the surroundings.
B) Heat flows from the surroundings to the system.
C) ( E = -35 kJ)
D) All of these are true.
E) None of these is true.
A) The system does work on the surroundings.
B) Heat flows from the surroundings to the system.
C) ( E = -35 kJ)
D) All of these are true.
E) None of these is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
14
Consider the following reaction: 2SO2(g) + O2(g) 2SO3(g) H = -198 kJ
Calculate the energy change associated with 21.4 g of SO2 reacting with excess O2.
A) -66.1 kJ
B) -4.24 *103 kJ
C) -33.1 kJ
D) -132 kJ
E) -198 kJ
Calculate the energy change associated with 21.4 g of SO2 reacting with excess O2.
A) -66.1 kJ
B) -4.24 *103 kJ
C) -33.1 kJ
D) -132 kJ
E) -198 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
15
Consider the following reaction: 2SO2(g) + O2(g) 2SO3(g) H = -198.2 kJ
Is the reaction endothermic or exothermic as written?
A) It is endothermic.
B) It is exothermic.
C) This can't be determined without more information.
Is the reaction endothermic or exothermic as written?
A) It is endothermic.
B) It is exothermic.
C) This can't be determined without more information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following statements is(are) true?
A) In exothermic reactions, the reactants are lower in potential energy than the products.
B) The heat of reaction and change in enthalpy can always be used interchangeably.
C) Enthalpy is a state function.
D) A chemist takes the point of view of the surroundings when determining the sign for work or heat.
E) At least two of these statements are true.
A) In exothermic reactions, the reactants are lower in potential energy than the products.
B) The heat of reaction and change in enthalpy can always be used interchangeably.
C) Enthalpy is a state function.
D) A chemist takes the point of view of the surroundings when determining the sign for work or heat.
E) At least two of these statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
17
For the reaction H2O(l) H2O(g) at 298 K, 1.0 atm, H is more positive than E by 2.5 kJ/mol. This quantity of energy can be considered to be
A) the heat flow required to maintain a constant temperature.
B) the work done in pushing back the atmosphere.
C) the value of H itself.
D) the difference in the H-O bond energy in H2O(l) compared to H2O(g).
E) none of these
A) the heat flow required to maintain a constant temperature.
B) the work done in pushing back the atmosphere.
C) the value of H itself.
D) the difference in the H-O bond energy in H2O(l) compared to H2O(g).
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which one of the following statements is false?
A) If qp for a process is negative, the process is exothermic.
B) A bomb calorimeter measures H directly.
C) The change in enthalpy, H, for a process is equal to the amount of heat absorbed at constant pressure, qp.
D) The freezing of water is an example of an exothermic reaction.
E) The change in internal energy, E, for a process is equal to the amount of heat absorbed at constant volume, qv.
A) If qp for a process is negative, the process is exothermic.
B) A bomb calorimeter measures H directly.
C) The change in enthalpy, H, for a process is equal to the amount of heat absorbed at constant pressure, qp.
D) The freezing of water is an example of an exothermic reaction.
E) The change in internal energy, E, for a process is equal to the amount of heat absorbed at constant volume, qv.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
19
Consider a gas in a 1.0-L bulb at STP that is connected via a valve to another bulb that is initially evacuated. Answer the following questions about what occurs when the valve between the two bulbs is opened.
What is true about the value of q?
A) It is equal to zero.
B) It is greater than zero.
C) It is less than zero.
What is true about the value of q?
A) It is equal to zero.
B) It is greater than zero.
C) It is less than zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
20
Given the equation S(s) + O2(g) SO2(g), H = -296 kJ, which of the following statements is(are) true?
I. The reaction is exothermic.
II. When 0.500 mol of sulfur is reacted, 148 kJ of energy is released.
III. When 32.0 g of sulfur is burned, 2.96 *105 J of energy is released.
A) I and III are true.
B) Only II is true.
C) All are true.
D) I and II are true.
E) None is true.
I. The reaction is exothermic.
II. When 0.500 mol of sulfur is reacted, 148 kJ of energy is released.
III. When 32.0 g of sulfur is burned, 2.96 *105 J of energy is released.
A) I and III are true.
B) Only II is true.
C) All are true.
D) I and II are true.
E) None is true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
21
A 22.6 g piece of aluminum (which has a molar heat capacity of 24.03 J/mol°C) is heated to 85.2°C and dropped into a calorimeter containing water (the specific heat capacity of water is 4.18 J/g°C) initially at 23.4°C. The final temperature of the water is 27.8°C. Calculate the mass of water in the calorimeter.
A) 6.3 *101 g
B) 1.7 *103 g
C) 0.37 g
D) 1.9 g
E) 6.8 * 101 g
A) 6.3 *101 g
B) 1.7 *103 g
C) 0.37 g
D) 1.9 g
E) 6.8 * 101 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
22
When 0.157 mol of NH3 is reacted with excess HCl, 6.91 kJ of energy is released as heat. What is H for this reaction per mole of NH3 consumed?
A) -22.7 J
B) -44.0 kJ
C) +44.0 kJ
D) -1.08 kJ
E) +22.7 J
A) -22.7 J
B) -44.0 kJ
C) +44.0 kJ
D) -1.08 kJ
E) +22.7 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
23
Two metals of equal mass with different heat capacities are subjected to the same amount of heat. Which undergoes the smaller change in temperature?
A) To determine this, you need to know which metals you are talking about.
B) Both undergo the same change in temperature.
C) The metal with the higher heat capacity.
D) The metal with the lower heat capacity.
E) To determine this, you need to know the initial temperatures of the metals.
A) To determine this, you need to know which metals you are talking about.
B) Both undergo the same change in temperature.
C) The metal with the higher heat capacity.
D) The metal with the lower heat capacity.
E) To determine this, you need to know the initial temperatures of the metals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
24
For the vaporization of water at 1.00 atm, H = 43.54 kJ/mol at 298 K and H = 40.68 kJ/mol at 373 K
The constant-pressure heat capacity of liquid water is 75.3 J/mol • K. Calculate the constant-pressure heat capacity for H2O(g).
A) 20.8 J/mol•K
B) 2790 J/mol•K
C) 75.3 J/mol•K
D) 37.2 J/mol•K
E) none of these
The constant-pressure heat capacity of liquid water is 75.3 J/mol • K. Calculate the constant-pressure heat capacity for H2O(g).
A) 20.8 J/mol•K
B) 2790 J/mol•K
C) 75.3 J/mol•K
D) 37.2 J/mol•K
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which statement is true of a process in which 1 mol of a gas is expanded from state A to state B?
A) The final volume of the gas will depend on the path taken.
B) The amount of work done in the process must be the same, regardless of the path.
C) When the gas expands from state A to state B, the surroundings are doing work on the system.
D) The amount of heat released in the process will depend on the path taken.
E) It is not possible to have more than one path for a change of state.
A) The final volume of the gas will depend on the path taken.
B) The amount of work done in the process must be the same, regardless of the path.
C) When the gas expands from state A to state B, the surroundings are doing work on the system.
D) The amount of heat released in the process will depend on the path taken.
E) It is not possible to have more than one path for a change of state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
26
Two samples of a monatomic ideal gas are in separate containers at the same conditions of pressure, volume, and temperature (V = 1.00 L and P = 1.00 atm). Both samples undergo changes in conditions and finish with V = 2.00 L and P = 2.00 atm. However, in the first sample, the volume is changed to 2.0 L while the pressure is kept constant, and then the pressure is increased to 2.00 atm while the volume remains constant. In the second sample, the opposite is done. The pressure is increased first, with constant volume, and then the volume is increased under constant pressure.
Calculate the difference in q between the first sample and the second sample.
A) 1.00 L•atm
B) -2.00 L•atm
C) 2.00 L•atm
D) -1.00 L•atm
E) none of these
Calculate the difference in q between the first sample and the second sample.
A) 1.00 L•atm
B) -2.00 L•atm
C) 2.00 L•atm
D) -1.00 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
27
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
Calculate qAB.
A) 135 L•atm
B) -135 L•atm
C) 225 L•atm
D) -225 L•atm
E) none of these
Calculate qAB.
A) 135 L•atm
B) -135 L•atm
C) 225 L•atm
D) -225 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
28
Two samples of a monatomic ideal gas are in separate containers at the same conditions of pressure, volume, and temperature (V = 1.00 L and P = 1.00 atm). Both samples undergo changes in conditions and finish with V = 2.00 L and P = 2.00 atm. However, in the first sample, the volume is changed to 2.0 L while the pressure is kept constant, and then the pressure is increased to 2.00 atm while the volume remains constant. In the second sample, the opposite is done. The pressure is increased first, with constant volume, and then the volume is increased under constant pressure.
Calculate the difference in w between the first sample and the second sample.
A) -2.00 L•atm
B) 1.00 L•atm
C) 2.00 L•atm
D) -1.00 L•atm
E) none of these
Calculate the difference in w between the first sample and the second sample.
A) -2.00 L•atm
B) 1.00 L•atm
C) 2.00 L•atm
D) -1.00 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider a gas in a 1.0-L bulb at STP that is connected via a valve to another bulb that is initially evacuated. Answer the following questions about what occurs when the valve between the two bulbs is opened.
-What is true about the value of H?
A) It is greater than zero.
B) It is less than zero.
C) It is equal to zero.
-What is true about the value of H?
A) It is greater than zero.
B) It is less than zero.
C) It is equal to zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
30
Consider a gas in a 1.0-L bulb at STP that is connected via a valve to another bulb that is initially evacuated. Answer the following questions about what occurs when the valve between the two bulbs is opened.
-What is true about the value of E?
A) It is less than zero.
B) It is greater than zero.
C) It is equal to zero.
-What is true about the value of E?
A) It is less than zero.
B) It is greater than zero.
C) It is equal to zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
31
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
Calculate wAC.
A) 0
B) 30 L•atm
C) -30 L•atm
D) 90 L•atm
E) -90 L•atm
Calculate wAC.
A) 0
B) 30 L•atm
C) -30 L•atm
D) 90 L•atm
E) -90 L•atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
32
A calorimeter contains 124 g of water at 27.0°C. A block of metal with a mass of 26 g is heated to 94.6°C and then placed in the water in the calorimeter. After sufficient time, the temperature of the water is measured and found to be 28.8°C. Calculate the heat capacity per gram of metal. Assume no heat is lost to the calorimeter or the surroundings.
A) 0.13 J/g°C
B) 7.3 *102 J/g°C
C) 0.024 J/g°C
D) 1.8 J/g°C
E) 0.55 J/g°C
A) 0.13 J/g°C
B) 7.3 *102 J/g°C
C) 0.024 J/g°C
D) 1.8 J/g°C
E) 0.55 J/g°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
33
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
Calculate wAB.
A) 0
B) 90 L•atm
C) -90 L•atm
D) -30 L•atm
E) 30 L•atm
Calculate wAB.
A) 0
B) 90 L•atm
C) -90 L•atm
D) -30 L•atm
E) 30 L•atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
34
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
If 5.0 kJ of energy is added to a 15.5-g sample of water at 10.°C, the water is
A) completely vaporized.
B) frozen solid.
C) boiling.
D) decomposed.
E) still a liquid.
If 5.0 kJ of energy is added to a 15.5-g sample of water at 10.°C, the water is
A) completely vaporized.
B) frozen solid.
C) boiling.
D) decomposed.
E) still a liquid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
35
You take 322 g of a solid (melting point = 57.2°C, heat of fusion = 349 J/g) and let it melt in 753 g of water. The water temperature decreases from its initial temperature to 57.2°C. Calculate the initial temperature of the water.
A) 92.9°C
B) 100.0°C
C) 21.5°C
D) 206.4°C
E) 252.4°C
A) 92.9°C
B) 100.0°C
C) 21.5°C
D) 206.4°C
E) 252.4°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
36
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
-Calculate HACD.
A) -175 L•atm
B) 175 L•atm
C) -25 L•atm
D) 25 L•atm
E) none of these
-Calculate HACD.
A) -175 L•atm
B) 175 L•atm
C) -25 L•atm
D) 25 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
37
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
-Calculate HABD.
A) -475 L•atm
B) -25 L•atm
C) 475 L•atm
D) 25 L•atm
E) none of these
-Calculate HABD.
A) -475 L•atm
B) -25 L•atm
C) 475 L•atm
D) 25 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
38
Consider a gas in a 1.0-L bulb at STP that is connected via a valve to another bulb that is initially evacuated. Answer the following questions about what occurs when the valve between the two bulbs is opened.
What is true about the value of w?
A) It is less than zero.
B) It is equal to zero.
C) It is greater than zero.
What is true about the value of w?
A) It is less than zero.
B) It is equal to zero.
C) It is greater than zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
39
Two samples of a monatomic ideal gas are in separate containers at the same conditions of pressure, volume, and temperature (V = 1.00 L and P = 1.00 atm). Both samples undergo changes in conditions and finish with V = 2.00 L and P = 2.00 atm. However, in the first sample, the volume is changed to 2.0 L while the pressure is kept constant, and then the pressure is increased to 2.00 atm while the volume remains constant. In the second sample, the opposite is done. The pressure is increased first, with constant volume, and then the volume is increased under constant pressure.
-Calculate the difference in E between the first sample and the second sample.
A) 0
B) 1.00 L•atm
C) 2.00 L•atm
D) 4.50 L•atm
E) none of these
-Calculate the difference in E between the first sample and the second sample.
A) 0
B) 1.00 L•atm
C) 2.00 L•atm
D) 4.50 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
40
Consider a process carried out on 1.00 mol of a monatomic ideal gas by the following two different pathways. The first pathway is A (3.00 atm, 20.0 L) to C (1.00 atm, 20.0 L) to D (1.00 atm, 50.0 L); and the second pathway is A (3.00 atm, 20.0 L) to B (3.00 atm, 50.0 L) to D (1.00 atm, 50.0 L). In each case, the gas is taken from state A to state D.
Calculate qAC.
A) 60 L•atm
B) 100 L•atm
C) -60 L•atm
D) -100 L•atm
E) none of these
Calculate qAC.
A) 60 L•atm
B) 100 L•atm
C) -60 L•atm
D) -100 L•atm
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
41
Using Hess's law and equations 1-3 below, find H° at 25°C for the oxidation of C2H5OH(l). C2H5OH(l) + 3O2(g) 3H2O(l) + 2CO2(g) 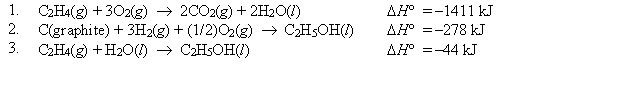
A) -1367 kJ
B) 44 kJ
C) 632 kJ
D) -1742 kJ
E) none of these
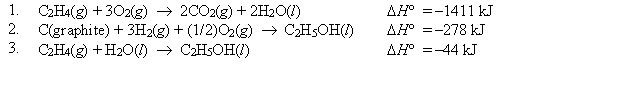
A) -1367 kJ
B) 44 kJ
C) 632 kJ
D) -1742 kJ
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
42
A calorimeter contains 95 g of water at 25.0°C. A 5.0-g sample of ice (at -5.0°C) is added to the water in the calorimeter, and eventually all of the ice melts. Calculate the final temperature of the water. Assume no heat is lost to the calorimeter or the surroundings.
A) 20.7°C
B) 21.2°C
C) 17.5°C
D) 19.6°C
E) none
A) 20.7°C
B) 21.2°C
C) 17.5°C
D) 19.6°C
E) none

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
43
A 50.0-g sample of a metal is heated to 98.7°C and then placed in a calorimeter containing 395.0 g of water (c = 4.18 J/g°C) at 22.5°C. The final temperature of the water is 24.5°C. Which metal was used?
A) iron (C = 0.45 J/g°C)
B) lead (C = 0.14 J/g°C)
C) copper (C = 0.20 J/g°C)
D) aluminum (C = 0.89 J/g°C)
E) none of these
A) iron (C = 0.45 J/g°C)
B) lead (C = 0.14 J/g°C)
C) copper (C = 0.20 J/g°C)
D) aluminum (C = 0.89 J/g°C)
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
44
The enthalpy of fusion of ice is 6.020 kJ/mol. The heat capacity of liquid water is 75.4 J/mol C. What is the smallest number of ice cubes at 0 C, each containing 1 mol of water, necessary to cool 500. g of liquid water initially at 20 C to 0 C?
A) 1
B) 15
C) 7
D) 14
E) 126
A) 1
B) 15
C) 7
D) 14
E) 126

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
45
One mole of a liquid is vaporized at its boiling point, 65°C and 1.00 atm. Hvap for the liquid is 43.8 kJ/mol at 65° C.
-Calculate E.
A) 71.5 kJ
B) 16.1 kJ
C) 41.0 kJ
D) 46.6 kJ
E) none of these
-Calculate E.
A) 71.5 kJ
B) 16.1 kJ
C) 41.0 kJ
D) 46.6 kJ
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
46
Calculate H° for the reaction C4H4(g) + 2H2(g) C4H8(g), using the following data: H°combustion for C4H4(g) = -2341 kJ/mol
H°combustion for H2(g) = -286 kJ/mol
H°combustion for C4H8(g) = -2755 kJ/mol
A) -128 kJ
B) 158 kJ
C) -158 kJ
D) 128 kJ
E) none of these
H°combustion for H2(g) = -286 kJ/mol
H°combustion for C4H8(g) = -2755 kJ/mol
A) -128 kJ
B) 158 kJ
C) -158 kJ
D) 128 kJ
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
47
A 140.0-g sample of water at 25.0°C is mixed with 100.0 g of a certain metal at 100.0°C. After thermal equilibrium is established, the (final) temperature of the mixture is 29.6°C. What is the heat capacity of the metal, assuming it is constant over the temperature range concerned?
A) 0.031 J/g°C
B) 0.38 J/g°C
C) 0.96 J/g°C
D) 0.76 J/g°C
E) none of these
A) 0.031 J/g°C
B) 0.38 J/g°C
C) 0.96 J/g°C
D) 0.76 J/g°C
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
48
At 25°C, the following heats of reaction are known: H (kJ/mol)
2ClF + O2 Cl2O + F2O
167.4
2ClF3 + 2O2 Cl2O + 3F2O
341.4
2F2 + O2 2F2O
-43.4
At the same temperature, calculate H for the following reaction:
A) -217.5 kJ/mol
B) -108.7 kJ/mol
C) +217.5 kJ/mol
D) -130.2 kJ/mol
E) none of these
2ClF + O2 Cl2O + F2O
167.4
2ClF3 + 2O2 Cl2O + 3F2O
341.4
2F2 + O2 2F2O
-43.4
At the same temperature, calculate H for the following reaction:

A) -217.5 kJ/mol
B) -108.7 kJ/mol
C) +217.5 kJ/mol
D) -130.2 kJ/mol
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
49
A calorimeter contains 143 g of water at 22.5°C. A 12 g sample of NaCl is added to the water in the calorimeter. After the solid has dissolved, the temperature of the water is 21.6°C. Calculate the enthalpy of solution for dissolving sodium chloride. Assume that no heat is lost to the calorimeter or the surroundings and that the specific heat of the solution is the same as that of pure water.
A) 0.049 kJ/mol
B) 0.58 kJ/mol
C) 0.68 kJ/mol
D) 2.6 kJ/mol
E) 2.8 kJ/mol
A) 0.049 kJ/mol
B) 0.58 kJ/mol
C) 0.68 kJ/mol
D) 2.6 kJ/mol
E) 2.8 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
50
Use the following table: Reaction
H° (kJ)
I. P4(s) + 6Cl2(g) 4PCl3(g)
-1225.6
II. P4(s) + 5O2(g) P4O10(g)
-2967.3
III. PCl3(g) + Cl2(g) PCl5(g)
-84.2
IV. PCl3(g) + (1/2)O2(g) Cl3PO(g)
-285.7
Calculate H° for the reaction
P4O10(g) + 6PCl5(g) 10Cl3PO(g)
A) -610.1 kJ
B) -2682.2 kJ
C) -7555.0 kJ
D) -110.5 kJ
E) None of these is within 5% of the correct answer.
H° (kJ)
I. P4(s) + 6Cl2(g) 4PCl3(g)
-1225.6
II. P4(s) + 5O2(g) P4O10(g)
-2967.3
III. PCl3(g) + Cl2(g) PCl5(g)
-84.2
IV. PCl3(g) + (1/2)O2(g) Cl3PO(g)
-285.7
Calculate H° for the reaction
P4O10(g) + 6PCl5(g) 10Cl3PO(g)
A) -610.1 kJ
B) -2682.2 kJ
C) -7555.0 kJ
D) -110.5 kJ
E) None of these is within 5% of the correct answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
51
A calorimeter contains 230 g of water at 21.4°C. A block of metal with a mass of 94 g is heated to 96.5°C and then placed in the water in the calorimeter. After sufficient time, the temperature of the water is measured and found to be 26.8°C. Calculate the specific heat capacity per gram of metal. Assume no heat is lost to the calorimeter or the surroundings.
A) 0.13 J/g°C
B) 0.032 J/g°C
C) 0.19 J/g°C
D) 0.79 J/g°C
E) 1.3 J/g°C
A) 0.13 J/g°C
B) 0.032 J/g°C
C) 0.19 J/g°C
D) 0.79 J/g°C
E) 1.3 J/g°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
52
Given: 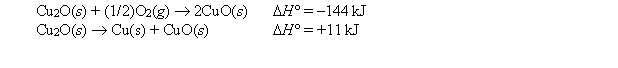 Calculate the standard enthalpy of formation of CuO(s).
Calculate the standard enthalpy of formation of CuO(s).
A) +299 kJ
B) +155 kJ
C) -166 kJ
D) -155 kJ
E) -299 kJ
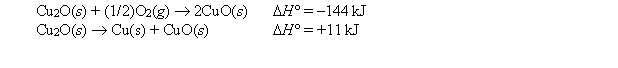 Calculate the standard enthalpy of formation of CuO(s).
Calculate the standard enthalpy of formation of CuO(s).A) +299 kJ
B) +155 kJ
C) -166 kJ
D) -155 kJ
E) -299 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
53
A 1.00-g sample of the rocket fuel hydrazine, N2H4, is burned in a bomb calorimeter containing 1200. g of water. The temperature of the water and the bomb calorimeter rises from 24.62°C to 28.16°C. Assuming the heat capacity of the empty bomb calorimeter is 837 J/°C, calculate the heat of combustion of 1 mol of hydrazine in the bomb calorimeter. (The specific heat capacity of water is 4.184 J/g•°C.)
A) -152 kJ
B) +47.4 kJ
C) +20.7 kJ
D) -665 kJ
E) -569 kJ
A) -152 kJ
B) +47.4 kJ
C) +20.7 kJ
D) -665 kJ
E) -569 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
54
Consider the following numbered processes:
1. A 2B
2. B C + D
3. E 2D
H for the process A 2C + E is
A) ( H1 + H2)
B) ( H1 + 2 H2 - H3)
C) ( H1 + H2 + H3)
D) ( H1 + 2 H2 + H3)
E) ( H1 + H2 - H3)
1. A 2B
2. B C + D
3. E 2D
H for the process A 2C + E is
A) ( H1 + H2)
B) ( H1 + 2 H2 - H3)
C) ( H1 + H2 + H3)
D) ( H1 + 2 H2 + H3)
E) ( H1 + H2 - H3)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
55
The standard enthalpy of formation of H2O2(l) at 298 K is -187.6 kJ/mol. Calculate the change in internal energy for the following process at 298 K: H2(g) + O2(g) H2O2(l)
A) -187.6 kJ/mol
B) 185.1 kJ/mol
C) -192.6 kJ/mol
D) -182.6 kJ/mol
E) -185.1 kJ/mol
A) -187.6 kJ/mol
B) 185.1 kJ/mol
C) -192.6 kJ/mol
D) -182.6 kJ/mol
E) -185.1 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
56
A bomb calorimeter has a heat capacity of 2.24 kJ/K. When a 0.170 g sample of gas with a molar mass of 28.0 g/mol was burned in this calorimeter, the temperature increased by 2.19 K. Calculate the energy of combustion for 1 mol of this gas.
A) -4.91 kJ
B) -0.0298 kJ
C) -8.08 *102 kJ
D) -1.37 * 102 kJ
E) -2.89 *101 kJ
A) -4.91 kJ
B) -0.0298 kJ
C) -8.08 *102 kJ
D) -1.37 * 102 kJ
E) -2.89 *101 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
57
75.0 mL of a pure liquid at 245 K is mixed with 100.0 mL of the same pure liquid at 365. K. What is the final temperature of the mixture?
A) 325 K
B) 295 K
C) 305 K
D) 314 K
E) none of these
A) 325 K
B) 295 K
C) 305 K
D) 314 K
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
58
At 25°C, the following heats of reaction are known: 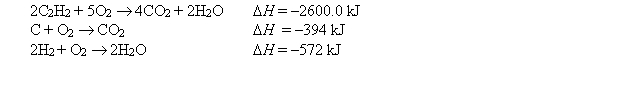 At the same temperature, calculate H for the following reaction:
At the same temperature, calculate H for the following reaction: 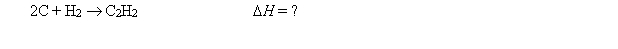
A) -2422kJ
B) -226 kJ
C) 226 kJ
D) 2422 kJ
E) none of these
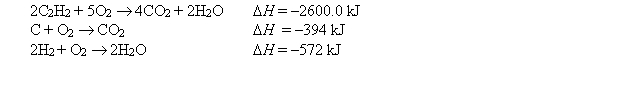 At the same temperature, calculate H for the following reaction:
At the same temperature, calculate H for the following reaction: 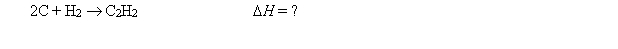
A) -2422kJ
B) -226 kJ
C) 226 kJ
D) 2422 kJ
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
59
One mole of a liquid is vaporized at its boiling point, 65°C and 1.00 atm. Hvap for the liquid is 43.8 kJ/mol at 65° C.
-Calculate w.
A) -27.7 J
B) 27.7 J
C) 2.81 * 103 J
D) -2.81 * 103 J
E) none of these
-Calculate w.
A) -27.7 J
B) 27.7 J
C) 2.81 * 103 J
D) -2.81 * 103 J
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
60
When a student performs an endothermic reaction in a calorimeter, how (if any) does the calculated value of H differ from the actual value if the heat exchanged with the calorimeter is not taken into account?
A) ( Hcalc) is less positive because the reaction absorbs heat from the calorimeter.
B) ( Hcalc) equals the actual value because the calorimeter does not absorb heat.
C) ( Hcalc) is more negative because the calorimeter always absorbs heat from the reaction.
D) ( Hcalc) is less negative because the calorimeter absorbs heat from the reaction.
E) ( Hcalc) is more positive because the reaction absorbs heat from the calorimeter.
A) ( Hcalc) is less positive because the reaction absorbs heat from the calorimeter.
B) ( Hcalc) equals the actual value because the calorimeter does not absorb heat.
C) ( Hcalc) is more negative because the calorimeter always absorbs heat from the reaction.
D) ( Hcalc) is less negative because the calorimeter absorbs heat from the reaction.
E) ( Hcalc) is more positive because the reaction absorbs heat from the calorimeter.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
61
For which of the following reaction(s) is the enthalpy change for the reaction not equal to H°f of the product?
I. 2H(g) H2(g)
II. H2(g) + O2(g) H2O2(l)
III. H2O(l) + O(g) H2O2(l)
A) I and III
B) III only
C) II and III
D) II only
E) I only
I. 2H(g) H2(g)
II. H2(g) + O2(g) H2O2(l)
III. H2O(l) + O(g) H2O2(l)
A) I and III
B) III only
C) II and III
D) II only
E) I only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
62
Using the information below, calculate H°f for PbO(s). PbO(s) + CO(g) Pb(s) + CO2(g), H° = -131.4 kJ
H°f for CO2(g) = -393.5 kJ/mol
H°f for CO(g) = -110.5 kJ/mol
A) +283.0 kJ/mol
B) +252.1 kJ/mol
C) -151.6 kJ/mol
D) -283.0 kJ/mol
E) -372.6 kJ/mol
H°f for CO2(g) = -393.5 kJ/mol
H°f for CO(g) = -110.5 kJ/mol
A) +283.0 kJ/mol
B) +252.1 kJ/mol
C) -151.6 kJ/mol
D) -283.0 kJ/mol
E) -372.6 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
63
The enthalpy of formation of an element in its standard state is
A) zero.
B) the enthalpy of its reaction with oxygen.
C) the enthalpy of its reaction with hydrogen.
D) determined by its melting point.
E) none of these
A) zero.
B) the enthalpy of its reaction with oxygen.
C) the enthalpy of its reaction with hydrogen.
D) determined by its melting point.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
64
Given the following two reactions at 298 K and 1 atm, which of the statements is true? 
A) ( Hf for NO2(g) = H2 + (1/2) H1)
B)( H1 = H2)
C) ( Hf for NO(g) = H10)
D) ( Hf for NO2(g) = H2)
E) none of these

A) ( Hf for NO2(g) = H2 + (1/2) H1)
B)( H1 = H2)
C) ( Hf for NO(g) = H10)
D) ( Hf for NO2(g) = H2)
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
65
The standard state of carbon as a free element is graphite. C60 is an allotropic form of carbon belonging to a class of structures known as fullerenes. 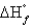 for C60 should be
for C60 should be
A) zero
B) positive
C) negative
D) equal to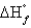 for the other allotropic forms of carbon
for the other allotropic forms of carbon
E) A and D
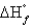 for C60 should be
for C60 should beA) zero
B) positive
C) negative
D) equal to
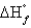 for the other allotropic forms of carbon
for the other allotropic forms of carbonE) A and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
66
Acetylene (C2H2) and butane (C4H10) are gaseous fuels. Determine the ratio of energy available from the combustion of a given volume of acetylene to butane at the same temperature and pressure using the following data:
The change in enthalpy of combustion for C2H2(g) = -49.9 kJ/g.
The change in enthalpy of combustion for C4H10 = -49.5 kJ/g.
The change in enthalpy of combustion for C2H2(g) = -49.9 kJ/g.
The change in enthalpy of combustion for C4H10 = -49.5 kJ/g.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
67
The combustion of methanol takes place according to the reaction 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l)
Calculate H for the combustion of 1 mol of methanol under standard conditions. Use the following standard enthalpies of formation: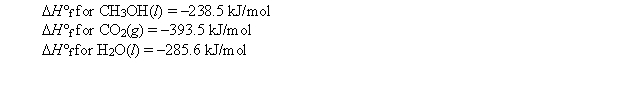
A) -1452.4 kJ/mol
B) +1452.4 kJ/mol
C) -726.2 kJ/mol
D) +726.2 kJ/mol
E) none of these
Calculate H for the combustion of 1 mol of methanol under standard conditions. Use the following standard enthalpies of formation:
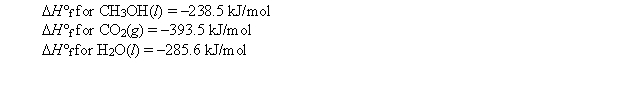
A) -1452.4 kJ/mol
B) +1452.4 kJ/mol
C) -726.2 kJ/mol
D) +726.2 kJ/mol
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
68
Specific heat capacities are tabulated on a
A) mass basis.
B) volume basis.
C) pressure basis.
D) mole basis.
A) mass basis.
B) volume basis.
C) pressure basis.
D) mole basis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
69
Using the following data, calculate the standard heat of formation of ICl(g) in kJ/mol. H° (kJ/mol)
Cl2(g) 2Cl(g)
242.3
I2 (g) 2I(g)
151.0
ICl(g) I(g) + Cl(g)
211.3
I2(s) I2(g)
62.8
A) 16.8 kJ/mol
B) -211 kJ/mol
C) 245 kJ/mol
D) -14.6 kJ/mol
E) 439 kJ/mol
Cl2(g) 2Cl(g)
242.3
I2 (g) 2I(g)
151.0
ICl(g) I(g) + Cl(g)
211.3
I2(s) I2(g)
62.8
A) 16.8 kJ/mol
B) -211 kJ/mol
C) 245 kJ/mol
D) -14.6 kJ/mol
E) 439 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
70
Consider the following data: 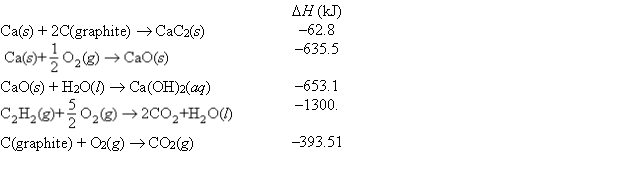 Use Hess's law to find the change in enthalpy at 25°C for the following equation:
Use Hess's law to find the change in enthalpy at 25°C for the following equation:
CaC2(s) + 2H2O(l) C2H2(g) + Ca(OH)2(aq)
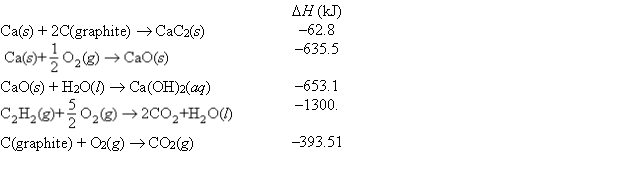 Use Hess's law to find the change in enthalpy at 25°C for the following equation:
Use Hess's law to find the change in enthalpy at 25°C for the following equation:CaC2(s) + 2H2O(l) C2H2(g) + Ca(OH)2(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
71
The heat of formation of Fe2O3(s) is -826 kJ/mol. Calculate the heat of the reaction 4Fe(s) + 3O2(g) 2Fe2O3(s) when a 18.6 g sample of iron is reacted.
A) -551 kJ
B) -138 kJ
C) -96.3 kJ
D) -826 kJ
E) -275 kJ
A) -551 kJ
B) -138 kJ
C) -96.3 kJ
D) -826 kJ
E) -275 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
72
Using the information below, calculate H°f for CH3OH(l). 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l), H° = -1453 kJ
H°f for CO2(g) = -393.5 kJ/mol
H°f for H2O(l) = -286 kJ/mol
A) 239 kJ/mol
B) -774 kJ/mol
C) -3.38 * 103 kJ/mol
D) 774 kJ/mol
E) -239 kJ/mol
H°f for CO2(g) = -393.5 kJ/mol
H°f for H2O(l) = -286 kJ/mol
A) 239 kJ/mol
B) -774 kJ/mol
C) -3.38 * 103 kJ/mol
D) 774 kJ/mol
E) -239 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following statements is true for a monatomic ideal gas?
A)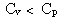
B)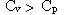
C)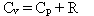
D)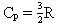
E)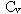 is temperature dependent
is temperature dependent
F) B and C
G) A, D , and E
A)
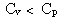
B)
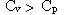
C)
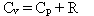
D)
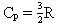
E)
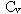 is temperature dependent
is temperature dependentF) B and C
G) A, D , and E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
74
Consider the following reaction:
2Al(s) + 3Cl2(g) 2AlCl3(s), H = -1390.81 kJ
A) Is the reaction exothermic or endothermic?
B) Calculate the heat produced when 10.0 g of AlCl3 forms.
C) How many grams of Al are required to produce 1.00 kJ of energy?
2Al(s) + 3Cl2(g) 2AlCl3(s), H = -1390.81 kJ
A) Is the reaction exothermic or endothermic?
B) Calculate the heat produced when 10.0 g of AlCl3 forms.
C) How many grams of Al are required to produce 1.00 kJ of energy?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
75
Choose the correct equation for the standard enthalpy of formation of CO(g), where H°f for CO = -110.5 kJ/mol.
A) Cgraphite(s) + CO2(g) 2CO(g), H° = -110.5 kJ
B) Cgraphite(s) + O(g) CO(g), H° = -110.5 kJ
C) 2Cgraphite(s) + O2(g) 2CO(g), H° = -110.5 kJ
D)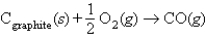 , H° = -110.5 kJ
, H° = -110.5 kJ
E) CO(g) Cgraphite(s) + O(g), H° = -110.5 kJ
A) Cgraphite(s) + CO2(g) 2CO(g), H° = -110.5 kJ
B) Cgraphite(s) + O(g) CO(g), H° = -110.5 kJ
C) 2Cgraphite(s) + O2(g) 2CO(g), H° = -110.5 kJ
D)
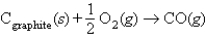 , H° = -110.5 kJ
, H° = -110.5 kJE) CO(g) Cgraphite(s) + O(g), H° = -110.5 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
76
The heat combustion of acetylene, C2H2(g), at 25°C, is -1299 kJ/mol. At this temperature, H°f values for CO2(g) and H2O(l) are -393 and -286 kJ/mol, respectively. Calculate H°f for acetylene.
A) 227 kJ/mol
B) -625 kJ/mol
C) 625 kJ/mol
D) 2376 kJ/mol
E) none of these
A) 227 kJ/mol
B) -625 kJ/mol
C) 625 kJ/mol
D) 2376 kJ/mol
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
77
For the reaction AgI(s) + (1/2)Br2(g) AgBr(s) + (1/2)I2(s), H° = -54.0 kJ
H°f for AgBr(s) = -100.4 kJ/mol
H°f for Br2(g) = +30.9 kJ/mol
The value of H°f for AgI(s) is
A) +61.8 kJ/mol
B) -77.3 kJ/mol
C) -61.8 kJ/mol
D) -123.5 kJ/mol
E) +77.3 kJ/mol
H°f for AgBr(s) = -100.4 kJ/mol
H°f for Br2(g) = +30.9 kJ/mol
The value of H°f for AgI(s) is
A) +61.8 kJ/mol
B) -77.3 kJ/mol
C) -61.8 kJ/mol
D) -123.5 kJ/mol
E) +77.3 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
78
Standard enthalpies of formation are tabulated on a
A) volume basis.
B) mass basis.
C) pressure basis.
D) mole basis.
A) volume basis.
B) mass basis.
C) pressure basis.
D) mole basis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
79
Consider the following standard heats of formation:
P4O10(s) = -3110 kJ/mol
H2O(l) = -286 kJ/mol
H3PO4(s) = -1279 kJ/mol
Calculate the change in enthalpy for the following process:
P4O10(s) + 6H2O(l) 4H3PO4(s)
P4O10(s) = -3110 kJ/mol
H2O(l) = -286 kJ/mol
H3PO4(s) = -1279 kJ/mol
Calculate the change in enthalpy for the following process:
P4O10(s) + 6H2O(l) 4H3PO4(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
80
The standard enthalpy change for the following reaction is -542 kJ: H2(g) + F2(g) 2HF(g)
Calculate the standard enthalpy of formation of hydrogen fluoride.
A) -1084 kJ/mol
B) 542 kJ/mol
C) -542 kJ/mol
D) -271 kJ/mol
E) none of these
Calculate the standard enthalpy of formation of hydrogen fluoride.
A) -1084 kJ/mol
B) 542 kJ/mol
C) -542 kJ/mol
D) -271 kJ/mol
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck








