Deck 2: Atoms, Molecules, and Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/61
العب
ملء الشاشة (f)
Deck 2: Atoms, Molecules, and Ions
1
Which of the following pairs of compounds can be used to illustrate the law of multiple proportions?
A) CaO and CaCl2
B) NO and NO2
C) H2O and HI
D) CH4 and CO2
E) NH3 and NBr3
A) CaO and CaCl2
B) NO and NO2
C) H2O and HI
D) CH4 and CO2
E) NH3 and NBr3
NO and NO2
2
Which are alkaline earth halides?
A) MgO, MgS, CaO
B) NaI, KBr, LiF
C) CaF2, MgBr2, SrI2
D) Al2O3, In2O3, Ga2S3
E) PbI2, PbBr2, CdF2
A) MgO, MgS, CaO
B) NaI, KBr, LiF
C) CaF2, MgBr2, SrI2
D) Al2O3, In2O3, Ga2S3
E) PbI2, PbBr2, CdF2
CaF2, MgBr2, SrI2
3
An element's most stable ion forms an ionic compound with chlorine having the formula XCl2. If the mass number of the ion is 24 and it has 10 electrons, what is the element and how many neutrons does it have?
A) Mg, 12 neutrons
B) Ne, 16 neutrons
C) O, 16 neutrons
D) Ne, 14 neutrons
E) Na, 11 neutrons
A) Mg, 12 neutrons
B) Ne, 16 neutrons
C) O, 16 neutrons
D) Ne, 14 neutrons
E) Na, 11 neutrons
Mg, 12 neutrons
4
Which one of the following statements about atomic structure is false?
A) Almost all of the mass of the atom is concentrated in the nucleus.
B) The protons and neutrons in the nucleus are very tightly packed.
C) The number of protons and the number of neutrons are always the same in the neutral atom.
D) The electrons occupy a very large volume compared to the nucleus.
A) Almost all of the mass of the atom is concentrated in the nucleus.
B) The protons and neutrons in the nucleus are very tightly packed.
C) The number of protons and the number of neutrons are always the same in the neutral atom.
D) The electrons occupy a very large volume compared to the nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following atomic symbols is incorrect?
A) (3115P)
B) (2010Ne)
C) (3417Cl)
D) (3919K)
E) (136N)
A) (3115P)
B) (2010Ne)
C) (3417Cl)
D) (3919K)
E) (136N)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which element does not belong to the family or classification indicated?
A) I, halogen
B) K, alkali metal
C) Sn, lanthanides
D) Ar, noble gas
E) Fe, transition metal
A) I, halogen
B) K, alkali metal
C) Sn, lanthanides
D) Ar, noble gas
E) Fe, transition metal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
7
The ion 31P3- has
A) 15 protons, 15 neutrons, 12 electrons
B) 15 protons, 15 neutrons, 3 electrons
C) 15 protons, 31 neutrons, 15 electrons
D) 15 protons, 16 neutrons, 18 electrons
E) 15 protons, 15 neutrons, 15 electrons
A) 15 protons, 15 neutrons, 12 electrons
B) 15 protons, 15 neutrons, 3 electrons
C) 15 protons, 31 neutrons, 15 electrons
D) 15 protons, 16 neutrons, 18 electrons
E) 15 protons, 15 neutrons, 15 electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which among the following represent a set of isotopes? Atomic nuclei containing
I. 20 protons and 20 neutrons.
II. 21 protons and 19 neutrons.
III. 22 neutrons and 18 protons.
IV. 20 protons and 22 neutrons.
V. 21 protons and 20 neutrons.
A) I, V
B) III, IV
C) I, II, III
D) I, IV and II, V
E) No isotopes are indicated.
I. 20 protons and 20 neutrons.
II. 21 protons and 19 neutrons.
III. 22 neutrons and 18 protons.
IV. 20 protons and 22 neutrons.
V. 21 protons and 20 neutrons.
A) I, V
B) III, IV
C) I, II, III
D) I, IV and II, V
E) No isotopes are indicated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
9
According to the law of definite proportions,
A) the ratio of the masses of the elements in a compound is always the same.
B) it is not possible for the same two elements to form more than one compound.
C) if the same two elements form two different compounds, they do so in the same ratio.
D) the total mass after a chemical change is the same as before the change.
A) the ratio of the masses of the elements in a compound is always the same.
B) it is not possible for the same two elements to form more than one compound.
C) if the same two elements form two different compounds, they do so in the same ratio.
D) the total mass after a chemical change is the same as before the change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the experiments listed below did not provide the information stated about the nature of the atom?
A) The Rutherford experiment proved that the Thomson "plum pudding" model of the atom was essentially correct.
B) The Rutherford experiment determined the charge on the nucleus.
C) The cathode-ray tube proved that electrons have a negative charge.
D) Millikan's oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.
A) The Rutherford experiment proved that the Thomson "plum pudding" model of the atom was essentially correct.
B) The Rutherford experiment determined the charge on the nucleus.
C) The cathode-ray tube proved that electrons have a negative charge.
D) Millikan's oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
11
The ion 127I- has
A) 53 protons, 74 neutrons, 52 electrons
B) 53 protons, 74 neutrons, 54 electrons
C) 53 protons, 53 neutrons, 53 electrons
D) 53 protons, 74 neutrons, 53 electrons
E) 53 protons, 127 neutrons, 54 electrons
A) 53 protons, 74 neutrons, 52 electrons
B) 53 protons, 74 neutrons, 54 electrons
C) 53 protons, 53 neutrons, 53 electrons
D) 53 protons, 74 neutrons, 53 electrons
E) 53 protons, 127 neutrons, 54 electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
12
The element rhenium (Re) exists as two stable isotopes and 18 unstable isotopes. Rhenium-185 has in its nucleus
A) 75 protons, 110 neutrons.
B) 75 protons, 75 neutrons.
C) 75 protons, 130 neutrons.
D) 130 protons, 75 neutrons.
E) not enough information is given.
A) 75 protons, 110 neutrons.
B) 75 protons, 75 neutrons.
C) 75 protons, 130 neutrons.
D) 130 protons, 75 neutrons.
E) not enough information is given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
13
When 3.0 L of hydrogen gas (H2) reacts with 1.0 L of nitrogen gas (N2), 2.0 L of gaseous product is formed. All volumes of gases are measured at the same temperature and pressure. What is the formula of the product?
A) NH
B) NH4
C) N2H3
D) N3H
E) NH3
A) NH
B) NH4
C) N2H3
D) N3H
E) NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following represents a pair of isotopes?
A) (157N, 158O)
B) (126C, 136C)
C) (188O, 199F)
D) (3216S, 3216S2-)
E) (O2, O3)
A) (157N, 158O)
B) (126C, 136C)
C) (188O, 199F)
D) (3216S, 3216S2-)
E) (O2, O3)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following statements is(are) true?
I. O and F have the same number of neutrons.
II. C and N are isotopes of each other because their mass numbers are the same.
III. O2- has the same number of electrons as Ne.
A) I only
B) II only
C) III only
D) I and II only
E) I and III only
I. O and F have the same number of neutrons.
II. C and N are isotopes of each other because their mass numbers are the same.
III. O2- has the same number of electrons as Ne.
A) I only
B) II only
C) III only
D) I and II only
E) I and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following statements is(are) true?
I. The number of protons is the same for all neutral atoms of an element.
II. The number of electrons is the same for all neutral atoms of an element.
III. The number of neutrons is the same for all neutral atoms of an element.
A) I, II, and III are all true.
B) I, II, and III are all false.
C) Only I and II are true.
D) Only I and III are true.
E) Only II and III are true.
I. The number of protons is the same for all neutral atoms of an element.
II. The number of electrons is the same for all neutral atoms of an element.
III. The number of neutrons is the same for all neutral atoms of an element.
A) I, II, and III are all true.
B) I, II, and III are all false.
C) Only I and II are true.
D) Only I and III are true.
E) Only II and III are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
17
How many of the following did Dalton not discuss in his atomic theory?
I. isotopes
II. ions
III. protons
IV. neutrons
V. electrons
A) 2
B) 5
C) 4
D) 1
E) 3
I. isotopes
II. ions
III. protons
IV. neutrons
V. electrons
A) 2
B) 5
C) 4
D) 1
E) 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
18
An ion is formed
I. by either adding protons to or subtracting protons from the atom.
II. by either adding electrons to or subtracting electrons from the atom.
III. by either adding neutrons to or subtracting neutrons from the atom.
A) Only I is true.
B) Only II is true.
C) Only III is true.
D) All of the statements are true.
E) Two of the statements are true.
I. by either adding protons to or subtracting protons from the atom.
II. by either adding electrons to or subtracting electrons from the atom.
III. by either adding neutrons to or subtracting neutrons from the atom.
A) Only I is true.
B) Only II is true.
C) Only III is true.
D) All of the statements are true.
E) Two of the statements are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which is the symbol for the isotope of nitrogen that has 7 protons and 8 neutrons?
A)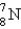
B)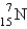
C)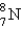
D)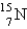
A)
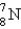
B)
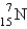
C)
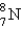
D)
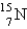

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
20
How many protons, neutrons, and electrons does the atom 31P have?
A) 16 protons, 15 neutrons, 16 electrons
B) 15 protons, 15 neutrons, 31 electrons
C) 16 protons, 16 neutrons, 15 electrons
D) 15 protons, 15 neutrons, 15 electrons
E) 15 protons, 16 neutrons, 15 electrons
A) 16 protons, 15 neutrons, 16 electrons
B) 15 protons, 15 neutrons, 31 electrons
C) 16 protons, 16 neutrons, 15 electrons
D) 15 protons, 15 neutrons, 15 electrons
E) 15 protons, 16 neutrons, 15 electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
21
Select the group of symbols that would correctly complete the following statements, respectively. ___ is the heaviest noble gas.
___ is the transition metal that has 24 electrons as a 3+ ion.
___ is the halogen in the third period.
___ is the alkaline earth metal that has 18 electrons as a stable ion.
A) Rn, Cr, Br, Ca
B) Ra, Sc, Br, K
C) Ra, Co, Cl, K
D) Rn, Co, Cl, Ca
___ is the transition metal that has 24 electrons as a 3+ ion.
___ is the halogen in the third period.
___ is the alkaline earth metal that has 18 electrons as a stable ion.
A) Rn, Cr, Br, Ca
B) Ra, Sc, Br, K
C) Ra, Co, Cl, K
D) Rn, Co, Cl, Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
22
Name the following compounds:
CCl4
CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following is not the correct chemical formula for the compound named?
A) Fe3SO4 iron(III) sulfate
B) BaBr2 barium bromide
C) Li2O lithium oxide
D) HCl hydrogen chloride
E) Mg3N2 magnesium nitride
A) Fe3SO4 iron(III) sulfate
B) BaBr2 barium bromide
C) Li2O lithium oxide
D) HCl hydrogen chloride
E) Mg3N2 magnesium nitride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which is not the correct chemical formula for the compound named?
A) iron(II) oxide FeO
B) potassium sulfate K2SO4
C) ammonium sulfide NH4S
D) zinc nitrate Zn(NO3)2
E) magnesium carbonate MgCO3
A) iron(II) oxide FeO
B) potassium sulfate K2SO4
C) ammonium sulfide NH4S
D) zinc nitrate Zn(NO3)2
E) magnesium carbonate MgCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which formula is not correct?
A) LiF
B) Ba(NO2)2
C) ZnBr
D) NaC2H3O2
E) CaO
A) LiF
B) Ba(NO2)2
C) ZnBr
D) NaC2H3O2
E) CaO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
26
Name the following compounds:
NH4NO3
NH4NO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
27
Complete the following table. 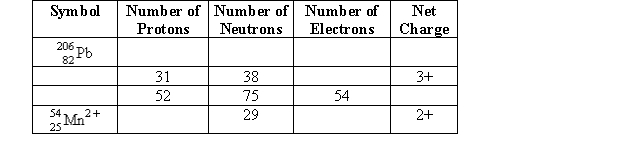


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
28
Name the following compounds:
K2Cr2O7
K2Cr2O7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
29
______ form ions with a 2+ charge when they react with nonmetals.
A) Halogens
B) Noble gases
C) Alkaline earth metals
D) Alkali metals
E) None of these choices
A) Halogens
B) Noble gases
C) Alkaline earth metals
D) Alkali metals
E) None of these choices

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following formulas is not correct?
A) Ba(OH)2
B) LiO
C) NaBr
D) CsCl
E) MgSO3
A) Ba(OH)2
B) LiO
C) NaBr
D) CsCl
E) MgSO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
31
Name the following compounds:
NaH
NaH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
32
Complete the following table. 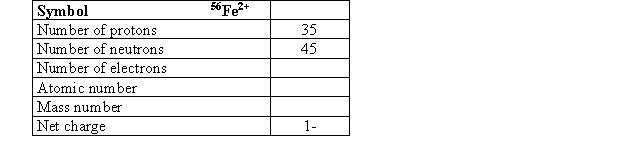


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following is not the correct chemical formula for the compound named?
A) HF hydrogen fluoride
B) MgO magnesium oxide
C) Fe3PO4 iron(III) phosphate
D) Li2O lithium oxide
E) BaCl2 barium chloride
A) HF hydrogen fluoride
B) MgO magnesium oxide
C) Fe3PO4 iron(III) phosphate
D) Li2O lithium oxide
E) BaCl2 barium chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following is not the correct name for the formula given?
A) HClO hypochlorous acid
B) Cr2O3 chromium(III) oxide
C) NCl3 nitrogen trichloride
D) CoO cobalt(II) oxide
E) CaSO4 calcium sulfite
A) HClO hypochlorous acid
B) Cr2O3 chromium(III) oxide
C) NCl3 nitrogen trichloride
D) CoO cobalt(II) oxide
E) CaSO4 calcium sulfite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following is not the correct chemical formula for the compound named?
A) Al(OH)2 aluminum hydroxide
B) Mg(C2H3O2)2 magnesium acetate
C) ZnS zinc sulfide
D) Fe2O3 iron(III) oxide
E) LiCN lithium cyanide
A) Al(OH)2 aluminum hydroxide
B) Mg(C2H3O2)2 magnesium acetate
C) ZnS zinc sulfide
D) Fe2O3 iron(III) oxide
E) LiCN lithium cyanide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which is the correct formula for gold(I) sulfide?
A) AuS
B) AuS2
C) Au2S2
D) Au2S
E) Au2S3
A) AuS
B) AuS2
C) Au2S2
D) Au2S
E) Au2S3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following is not the correct name for the formula given?
A) PCl5 phosphorus pentachoride
B) Fe2O3 iron(III) oxide
C) HClO hypochlorous acid
D) BaSO3 barium sulfate
E) CoO cobalt(II) oxide
A) PCl5 phosphorus pentachoride
B) Fe2O3 iron(III) oxide
C) HClO hypochlorous acid
D) BaSO3 barium sulfate
E) CoO cobalt(II) oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the correct formula for chromium(VI) oxide?
A) CrO6
B) CrO2
C) Cr2O3
D) Cr6O
E) CrO3
A) CrO6
B) CrO2
C) Cr2O3
D) Cr6O
E) CrO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
39
Name the following compounds:
Al2(SO4)3
Al2(SO4)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the correct formula for barium phosphate?
A) Ba2PO4
B) Ba3(PO4)2
C) Ba2(PO4)3
D) Ba3PO4
E) BaPO4
A) Ba2PO4
B) Ba3(PO4)2
C) Ba2(PO4)3
D) Ba3PO4
E) BaPO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
41
Name the following compounds:
CaSO4
CaSO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which nuclide has more protons than neutrons?
A)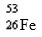
B)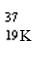
C)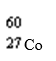
D)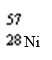
A)
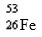
B)
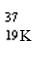
C)
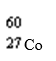
D)
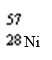

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
43
Name the following compounds:
HNO3
HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
44
Write the names of the following compounds:
A) FeSO4__________________________________________
B)NaC2H3O2__________________________________________
C) KNO2__________________________________________
D) Ca(OH)2__________________________________________
E) NiCO3__________________________________________
A) FeSO4__________________________________________
B)NaC2H3O2__________________________________________
C) KNO2__________________________________________
D) Ca(OH)2__________________________________________
E) NiCO3__________________________________________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
45
An isotope of an element is formed
I. by adding protons to, or removing protons from, the atom.
II. by adding neutrons to, or removing neutrons from, the atom.
III. by adding electrons to, or removing electrons from, the atom.
A) Only I is true
B) Only II is true
C) Only III is true
D) All of the statements are true
E) Two of the statements are true
I. by adding protons to, or removing protons from, the atom.
II. by adding neutrons to, or removing neutrons from, the atom.
III. by adding electrons to, or removing electrons from, the atom.
A) Only I is true
B) Only II is true
C) Only III is true
D) All of the statements are true
E) Two of the statements are true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
46
Write the formula for:
sulfurous acid
sulfurous acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
47
Name the following compounds:
N2O3
N2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
48
Write the formula for:
aluminum hydroxide
aluminum hydroxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
49
Write the chemical formulas for the following compounds or ions.
A) nitrate ion_________________
B) aluminum oxide_________________
C) ammonium ion_________________
D) perchloric acid_________________
E) copper(II) bromide_________________
A) nitrate ion_________________
B) aluminum oxide_________________
C) ammonium ion_________________
D) perchloric acid_________________
E) copper(II) bromide_________________

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
50
Write the formula for:
cobalt(II) chloride
cobalt(II) chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
51
Write the formula for:
phosphoric acid
phosphoric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
52
Write the formula for:
sodium dichromate
sodium dichromate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
53
Write the formula for:
iron(III) oxide
iron(III) oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
54
Write the formula for:
nitric acid
nitric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
55
Write the formula for:
dinitrogen trioxide
dinitrogen trioxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
56
Name the following compounds:
SnI2
SnI2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
57
Write the formula for:
hydrosulfuric acid
hydrosulfuric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
58
Name the following compounds:
AgCl
AgCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
59
Write the formula for:
acetic acid
acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which statement or statements regarding Antoine Lavoisier and his discovery of the conservation of mass in chemical reactions must be false.
A) Lavoisier conducted his experiment in an apparatus that trapped all reaction products.
B) Lavoisier was able to make accurate mass measurements.
C) Lavoisier was able to make precise mass measurements.
D) Lavoisier did not trap gases in his experiments because their mass was negligible.
E) A and D
A) Lavoisier conducted his experiment in an apparatus that trapped all reaction products.
B) Lavoisier was able to make accurate mass measurements.
C) Lavoisier was able to make precise mass measurements.
D) Lavoisier did not trap gases in his experiments because their mass was negligible.
E) A and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck
61
The experiments of what two scientists were instrumental in determining the mass and charge of the electron?
A) Lavoisier and Dalton
B) Rutherford and Curie
C) Thompson and Rutherford
D) Millikan and Cannizzaro
E) Thompson and Millikan
A) Lavoisier and Dalton
B) Rutherford and Curie
C) Thompson and Rutherford
D) Millikan and Cannizzaro
E) Thompson and Millikan

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 61 في هذه المجموعة.
فتح الحزمة
k this deck








