Deck 12: Alkenes, Alkynes, and Aromatic Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/81
العب
ملء الشاشة (f)
Deck 12: Alkenes, Alkynes, and Aromatic Compounds
1
The compound 1-butyne contains
A) all single bonds.
B) a double bond.
C) a triple bond.
D) a ring structure.
E) a bromine atom.
A) all single bonds.
B) a double bond.
C) a triple bond.
D) a ring structure.
E) a bromine atom.
a triple bond.
2
Which of the compounds is a cycloalkene?
A) CH₂ = CHCH = CH₂
B)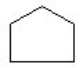
C) CH3C = CH₂
D)
E)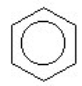
A) CH₂ = CHCH = CH₂
B)

C) CH3C = CH₂
D)

E)


3
What is the condensed structural formula of the compound propene?
A) CH3CH₂CH3
B) H3C = CH₂CH3
C) H₂C = C = CH₂
D) CH3CH = CH₂
E) HC ≡ CCH3
A) CH3CH₂CH3
B) H3C = CH₂CH3
C) H₂C = C = CH₂
D) CH3CH = CH₂
E) HC ≡ CCH3
CH3CH = CH₂
4
What is the IUPAC name for the following compound? 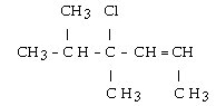
A) 4-chloro-4,5-dimethyl-2-hexene
B) 3-chloro-1,3,4-trimethyl-1-pentene
C) 3-chloro-2,3-dimethyl-4-hexene
D) 3-chloro-2,3,5-trimethyl-4-pentene
E) 3-chloro-1,3,4,4-tetramethyl-1-butene
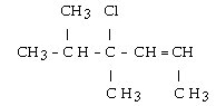
A) 4-chloro-4,5-dimethyl-2-hexene
B) 3-chloro-1,3,4-trimethyl-1-pentene
C) 3-chloro-2,3-dimethyl-4-hexene
D) 3-chloro-2,3,5-trimethyl-4-pentene
E) 3-chloro-1,3,4,4-tetramethyl-1-butene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following compounds is an alkyne?
A) CH3CH₂CH3
B) C₃H6
C) CH3CH₂C ≡ CH
D) H₂C = CHCH = CH₂
E) 2-pentene
A) CH3CH₂CH3
B) C₃H6
C) CH3CH₂C ≡ CH
D) H₂C = CHCH = CH₂
E) 2-pentene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
6
A hydrocarbon with a double bond is a(n)
A) alkane.
B) alkene.
C) alkyne.
D) alcohol.
E) saturated compound.
A) alkane.
B) alkene.
C) alkyne.
D) alcohol.
E) saturated compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
7
The IUPAC name for ethylene is
A) ethane.
B) cycloethane.
C) ethyne.
D) ethanene.
E) ethene.
A) ethane.
B) cycloethane.
C) ethyne.
D) ethanene.
E) ethene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
8
Name the following compound. 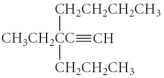
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne
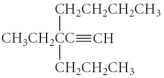
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
9
The IUPAC name for CH3CH₂C ≡ CCH3 is
A) 3-pentyne.
B) 2-pentyne.
C) pentyne.
D) 1-methylbutyne.
E) 2-propene.
A) 3-pentyne.
B) 2-pentyne.
C) pentyne.
D) 1-methylbutyne.
E) 2-propene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
10
Name the following compound. 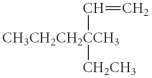
A) 2,2-diethylpenatane
B) 2,2-diethylpentene
C) 4-ethyl-4-methyl-5-hexene
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane
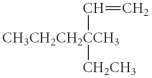
A) 2,2-diethylpenatane
B) 2,2-diethylpentene
C) 4-ethyl-4-methyl-5-hexene
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which one of the following compounds has the smallest number of hydrogen atoms?
A) butyne
B) 2-methylpropane
C) butene
D) 2-methylcyclopropane
E) butane
A) butyne
B) 2-methylpropane
C) butene
D) 2-methylcyclopropane
E) butane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
12
When naming an alkene, the parent chain is the longest carbon chain
A) that does not contain the double bond.
B) regardless of whether or not it contains the double bond.
C) that contains at least one of the carbon atoms of the double bond.
D) that contains both atoms of the double bond.
E) that contains a branch.
A) that does not contain the double bond.
B) regardless of whether or not it contains the double bond.
C) that contains at least one of the carbon atoms of the double bond.
D) that contains both atoms of the double bond.
E) that contains a branch.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
13
Alkenes and alkynes are called unsaturated compounds because
A) they have the maximum number of hydrogen atoms attached to each carbon in the compound.
B) they have fewer hydrogen atoms attached to the carbon chain than alkanes.
C) they have more hydrogen atoms attached to the carbon chain than alkanes.
D) they have more carbon atoms than alkanes.
E) they have fewer carbon atoms than alkanes.
A) they have the maximum number of hydrogen atoms attached to each carbon in the compound.
B) they have fewer hydrogen atoms attached to the carbon chain than alkanes.
C) they have more hydrogen atoms attached to the carbon chain than alkanes.
D) they have more carbon atoms than alkanes.
E) they have fewer carbon atoms than alkanes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
14
The carbon atoms in saturated hydrocarbons
A) have only single bonds.
B) contain at least one double bond.
C) contain at least one triple bond.
D) contain a benzene ring.
E) contain both a double and a triple bond.
A) have only single bonds.
B) contain at least one double bond.
C) contain at least one triple bond.
D) contain a benzene ring.
E) contain both a double and a triple bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
15
Organic compounds with double or triple bonds are classified as
A) unsaturated compounds.
B) saturated compounds.
C) dilute solutions.
D) concentrated solutions.
E) substituted compounds.
A) unsaturated compounds.
B) saturated compounds.
C) dilute solutions.
D) concentrated solutions.
E) substituted compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is the IUPAC name for the compound below? 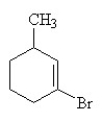
A) m-bromotoluene
B) m-bromomethylcyclohexene
C) 1-bromo-3-methylcyclohexene
D) 3-bromo-1-methyl-2-cyclohexene
E) 2-bromo-6-methylcyclohexene

A) m-bromotoluene
B) m-bromomethylcyclohexene
C) 1-bromo-3-methylcyclohexene
D) 3-bromo-1-methyl-2-cyclohexene
E) 2-bromo-6-methylcyclohexene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the condensed structural formula for the compound 3-hexene?
A)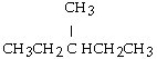
B) CH₂= CHCH₂CH₂CH₂CH3
C) CH3CH₂CH = CHCH₂CH3
D) CH3CH₂CH₂CH = CHCH3
E) CH3CH = CHCH₂CH₂CH3
A)
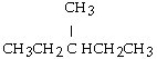
B) CH₂= CHCH₂CH₂CH₂CH3
C) CH3CH₂CH = CHCH₂CH3
D) CH3CH₂CH₂CH = CHCH3
E) CH3CH = CHCH₂CH₂CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following names are correct?
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
19
The IUPAC name of CH3CH = CHCH3 is
A) 2-butene.
B) 2-butane.
C) 1-butene.
D) butene.
E) 2-butyne.
A) 2-butene.
B) 2-butane.
C) 1-butene.
D) butene.
E) 2-butyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
20
An unsaturated compound always
A) is a cycloalkane.
B) contains a double bond.
C) contains a triple bond.
D) contains at least one double or triple bond.
E) is aromatic.
A) is a cycloalkane.
B) contains a double bond.
C) contains a triple bond.
D) contains at least one double or triple bond.
E) is aromatic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following pairs of compounds are cis-trans isomers?
A)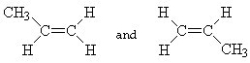
B)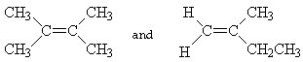
C) HC ≡ C - CH3 and CH3- C ≡ CH
D)
E)
A)
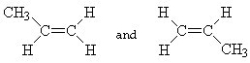
B)
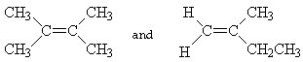
C) HC ≡ C - CH3 and CH3- C ≡ CH
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
22
The chemical reaction of 2-butene and HCl yields what product?
A) CH3CH₂CH₂CH3
B)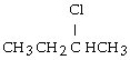
C)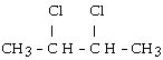
D)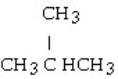
E)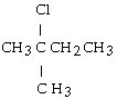
A) CH3CH₂CH₂CH3
B)
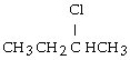
C)
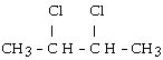
D)
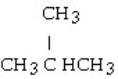
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
23
According to Markovnikov's rule, the hydrogen in HCl adds to the carbon in the double bond
A) attached to the end carbon.
B) that has the smaller number of hydrogen atoms attached.
C) that has the greater number of hydrogen atoms attached.
D) that has the smaller number of carbon atoms attached.
E) that has the greater number of carbon atoms attached.
A) attached to the end carbon.
B) that has the smaller number of hydrogen atoms attached.
C) that has the greater number of hydrogen atoms attached.
D) that has the smaller number of carbon atoms attached.
E) that has the greater number of carbon atoms attached.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
24
The reaction of hydrogen (H₂) and propene using a platinum catalyst is called
A) combustion.
B) substitution.
C) addition.
D) neutralization.
E) condensation.
A) combustion.
B) substitution.
C) addition.
D) neutralization.
E) condensation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following is the correct structural formula for 3-methylcyclohexene?
A)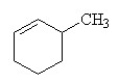
B)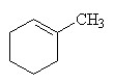
C)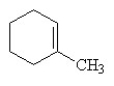
D)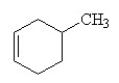
E)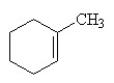
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
26
Insects communicate with chemicals called
A) markers.
B) isomers.
C) signals.
D) pheromones.
E) scents.
A) markers.
B) isomers.
C) signals.
D) pheromones.
E) scents.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
27
The reaction of an alkene and water in the presence of an acid catalyst to produce an alcohol is called
A) hydrolysis.
B) alkoholysis.
C) halogenation.
D) hydration.
E) hydrohydration.
A) hydrolysis.
B) alkoholysis.
C) halogenation.
D) hydration.
E) hydrohydration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the name of the compound shown below? 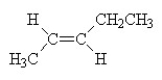
A) 2-pentene
B) trans-2-pentene
C) trans-3-pentene
D) cis-2-pentene
E) cis-3-pentene

A) 2-pentene
B) trans-2-pentene
C) trans-3-pentene
D) cis-2-pentene
E) cis-3-pentene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
29
The hydrogenation of an alkene gives a(n).
A) alkane.
B) alkene.
C) alkyne.
D) benzene.
E) isomer.
A) alkane.
B) alkene.
C) alkyne.
D) benzene.
E) isomer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
30
Some alkenes have cis-trans isomers because
A) the carbon atoms in the double bond cannot rotate.
B) each of the carbon atoms in the double bond has four different groups attached to it.
C) one of the carbon atoms in the double bond has two identical groups attached to it.
D) the carbon atoms in the double bond are free to rotate.
E) all of the carbon atoms in the compound are rigid and cannot rotate.
A) the carbon atoms in the double bond cannot rotate.
B) each of the carbon atoms in the double bond has four different groups attached to it.
C) one of the carbon atoms in the double bond has two identical groups attached to it.
D) the carbon atoms in the double bond are free to rotate.
E) all of the carbon atoms in the compound are rigid and cannot rotate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
31
The reactants needed to produce the compound chlorocyclopentane are
A) pentene and HCl.
B) pentene and Cl2
C) cyclopentane and Cl2
D) cyclopentene and HCl.
E) cyclopentene and Cl2
A) pentene and HCl.
B) pentene and Cl2
C) cyclopentane and Cl2
D) cyclopentene and HCl.
E) cyclopentene and Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
32
The major product of the reaction of 3-methyl-2-pentene with HCl is
A) 4-chloro-3-methylpentane.
B) 1-chloro-3-methylpentane.
C) 2-chloro-3-methylpentane.
D) 3-chloro-3-methylpentane.
E) 3-chloro-3-methylpentene.
A) 4-chloro-3-methylpentane.
B) 1-chloro-3-methylpentane.
C) 2-chloro-3-methylpentane.
D) 3-chloro-3-methylpentane.
E) 3-chloro-3-methylpentene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following compounds exhibit geometric isomerism?
A) CH₂=CH-CH3
B) CCl₂=CBr₂
C) CH3-CH=CH-CH3
D) CCl₂=CHBr
E) All of the above exhibit geometric isomerism.
A) CH₂=CH-CH3
B) CCl₂=CBr₂
C) CH3-CH=CH-CH3
D) CCl₂=CHBr
E) All of the above exhibit geometric isomerism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
34
The reaction of propene and Br₂ yields
A) 1-bromopropane.
B) 2-bromopropane.
C) 1,1-dibromopropane.
D) 1,2-dibromopropane.
E) 2,2-dibromopropane.
A) 1-bromopropane.
B) 2-bromopropane.
C) 1,1-dibromopropane.
D) 1,2-dibromopropane.
E) 2,2-dibromopropane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst?
A) CH3CH = CHCH3
B) Cl |
CH3CH₂CHCH3
C) CH3CH₂CH₂CH3
D)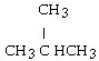
E)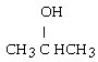
A) CH3CH = CHCH3
B) Cl |
CH3CH₂CHCH3
C) CH3CH₂CH₂CH3
D)
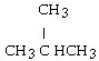
E)
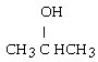

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the major product of the reaction shown below? CH3- CH₂- CH = CH₂ + HOH 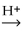
A)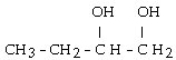
B)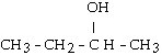
C) CH3-CH₂-CH₂-CH₂- OH
D)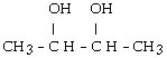
E)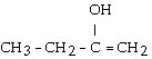
A)
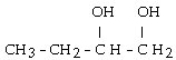
B)
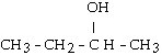
C) CH3-CH₂-CH₂-CH₂- OH
D)
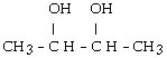
E)
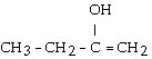

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the product of this reaction? CH3CH₂CH = CHCH3 + Cl₂ → ?
A) CH3CH₂CH₂CH₂CH3
B)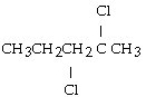
C)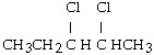
D)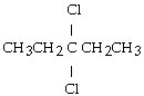
E)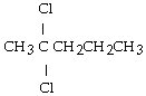
A) CH3CH₂CH₂CH₂CH3
B)
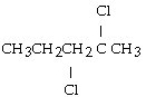
C)
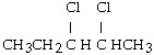
D)

E)
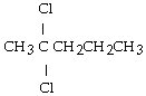

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
38
The reaction of cyclohexene and Cl₂ yields
A) 1-chlorocyclohexene.
B) 1,2-dichlorocyclohexane.
C) 1,3-dichlorocyclohexane.
D) 2,3-dichlorocyclohexane.
E) 3,4-dichlorocyclohexane.
A) 1-chlorocyclohexene.
B) 1,2-dichlorocyclohexane.
C) 1,3-dichlorocyclohexane.
D) 2,3-dichlorocyclohexane.
E) 3,4-dichlorocyclohexane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
39
The odors you associate with lemons, oranges, roses, and lavender are due to
A) alkenes.
B) alkanes.
C) alkynes.
D) thiols.
E) amines.
A) alkenes.
B) alkanes.
C) alkynes.
D) thiols.
E) amines.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which set of reactants would give this compound? 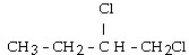
A) CH3CH?CH?CH3 + 2HCl
B) CH3CH CHCH3 + 2HCl
C) CH3CH?CH CH? + 2HCl
D) CH3CH CHCH3 + Cl?
E) CH3CH?CH CH? + Cl?
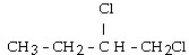
A) CH3CH?CH?CH3 + 2HCl
B) CH3CH CHCH3 + 2HCl
C) CH3CH?CH CH? + 2HCl
D) CH3CH CHCH3 + Cl?
E) CH3CH?CH CH? + Cl?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
41
A compound that contains the ring structure of benzene is called a(n)
A) alkane.
B) cycloalkane.
C) alkyl group.
D) aromatic compound.
E) hydrocarbon.
A) alkane.
B) cycloalkane.
C) alkyl group.
D) aromatic compound.
E) hydrocarbon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
42
The compound below is named 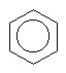
A) cyclohexane.
B) cyclohexene.
C) cyclohexyne.
D) benzene.
E) cyclobenzene.

A) cyclohexane.
B) cyclohexene.
C) cyclohexyne.
D) benzene.
E) cyclobenzene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
43
How many isomers are there for dibromobenzene?
A) one
B) two
C) three
D) four
E) five
A) one
B) two
C) three
D) four
E) five

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
44
Small molecules that make up the repeat unit in polymers are called
A) monomers.
B) alkenes.
C) alkynes.
D) minipolymers.
E) synthetic polymers.
A) monomers.
B) alkenes.
C) alkynes.
D) minipolymers.
E) synthetic polymers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
45
Alkynes contain double bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
46
The name of the compound shown below is 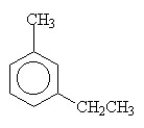
A) o-ethylmethylcyclohexane.
B) m-ethylmethylcyclohexane.
C) o-ethyltoluene.
D) m-ethyltoluene.
E) p-ethyltoluene.

A) o-ethylmethylcyclohexane.
B) m-ethylmethylcyclohexane.
C) o-ethyltoluene.
D) m-ethyltoluene.
E) p-ethyltoluene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
47
The structural formula of benzene is often represented as a
A) ring of five carbon atoms.
B) ring of six carbon atoms with six double bonds.
C) ring of six carbon atoms with a circle in the center.
D) cycloalkane.
E) cycloalkyne.
A) ring of five carbon atoms.
B) ring of six carbon atoms with six double bonds.
C) ring of six carbon atoms with a circle in the center.
D) cycloalkane.
E) cycloalkyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following is a naturally occurring polymer (not a synthetic polymer)?
A) polypropylene
B) DNA
C) teflon
D) polystyrene
E) saran
A) polypropylene
B) DNA
C) teflon
D) polystyrene
E) saran

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
49
All of the carbon-carbon bonds in benzene are
A) composed of only two types, single and double.
B) identical.
C) double bonds.
D) single bonds.
E) circular bonds.
A) composed of only two types, single and double.
B) identical.
C) double bonds.
D) single bonds.
E) circular bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
50
What are the prefixes used to designate substituent positions on disubstituted benzene compounds?
A) ortho-, meta-, and para-.
B) cis- and trans-
C) syn- and anti-
D) All of the above.
E) None of the above.
A) ortho-, meta-, and para-.
B) cis- and trans-
C) syn- and anti-
D) All of the above.
E) None of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following names are correct?
A) 4-bromo-2-ethylbenzene
B) 1-chloro-3-propylbenzene
C) 3-bromo-4-chlorobenzene
D) 2,2-dibromobenzene
E) 4-fluoro-2-iodobenzene
A) 4-bromo-2-ethylbenzene
B) 1-chloro-3-propylbenzene
C) 3-bromo-4-chlorobenzene
D) 2,2-dibromobenzene
E) 4-fluoro-2-iodobenzene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
52
Propylene is used to induce ripening in fruits.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
53
What is the starting monomer for the polymer Teflon? 
A)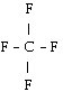
B)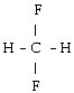
C)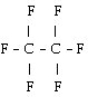
D) C F
E)

A)
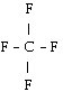
B)

C)

D) C F
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
54
When chlorine atoms are attached to carbon 1 and carbon 3 in benzene, the compound is named
A) dichlorobenzene.
B) o-dichlorobenzene.
C) m-dichlorobenzene.
D) p-dichlorobenzene.
E) j-dichlorobenzene.
A) dichlorobenzene.
B) o-dichlorobenzene.
C) m-dichlorobenzene.
D) p-dichlorobenzene.
E) j-dichlorobenzene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
55
The synthetic polymer polyethylene is unreactive because it is
A) unsaturated.
B) a haloalkane.
C) saturated.
D) a cycloalkene.
E) an aromatic compound.
A) unsaturated.
B) a haloalkane.
C) saturated.
D) a cycloalkene.
E) an aromatic compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the molecular formula of benzene?
A) C6H4
B) C6H6
C) C6H₈
D) C6H10
E) C6H12
A) C6H4
B) C6H6
C) C6H₈
D) C6H10
E) C6H12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
57
When bromine atoms are attached to carbon 1 and carbon 4 in benzene, the compound is named
A) dibromobenzene.
B) o-dibromobenzene.
C) m-dibromobenzene.
D) p-dibromobenzene.
E) f-dibromobenzene.
A) dibromobenzene.
B) o-dibromobenzene.
C) m-dibromobenzene.
D) p-dibromobenzene.
E) f-dibromobenzene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the name of the compound below? 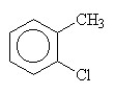
A) p-chlorotoluene
B) o-chlorotoluene
C) m-chlorotoluene
D) 1-chloro-2-methyltoluene
E) 2-chloro-1-methyltoluene

A) p-chlorotoluene
B) o-chlorotoluene
C) m-chlorotoluene
D) 1-chloro-2-methyltoluene
E) 2-chloro-1-methyltoluene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following would result from the polymerization of ethene?
A)
B)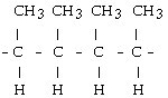
C)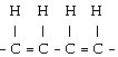
D) = C = C = C = C =
E) - CH₂- CH₂- CH₂- CH₂-
A)

B)
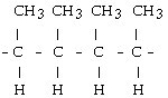
C)
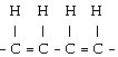
D) = C = C = C = C =
E) - CH₂- CH₂- CH₂- CH₂-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
60
Long-chain molecules that consist of many repeating units are called
A) polymers.
B) monomers.
C) organic compounds.
D) alkenes.
E) alkanes.
A) polymers.
B) monomers.
C) organic compounds.
D) alkenes.
E) alkanes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
61
In a cis alkene, the groups are on the same side of the double bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
62
The substituent group C6H5CH₂- is called a phenyl group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
63
Ortho compounds have substituents on the 1 and 3 positions of the benzene ring.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
64
Water can be added to alkenes to produce acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
65
Markovnikov's rule states that when a hydrogen halide adds to a double bond, the proton will bond to the carbon that has the greater number of hydrogen atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
66
Fragrances and flavors are often compounds with more than one functional group (for example, an alkene that also contains an aldehyde).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
67
Polymers are large molecules consisting of repeating units.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
68
One essential building block of aspirin, ibuprofen, and acetaminophen is the benzene ring.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
69
Halogenation is the process of adding chlorine or similar elements to an alkene or alkyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
70
Para compounds have substituents on the 1 and 4 positions of the benzene ring.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
71
Light-induced cis-trans isomerization is an important step in vision.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
72
Nylon, polyester, and most other plastics are carbon compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
73
Hydrogenation of unsaturated vegetable oils raises the melting point and makes them more solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
74
In a trans alkene, the groups are on the same side of the double bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
75
Hydration is used to convert alkenes and alkynes to alkanes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
76
Most products made from polymers can be recycled.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
77
Alkynes can show cis-trans isomerism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
78
An alkynes containing three carbons is named propyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
79
Hydrogenation is used to convert alkenes and alkynes to alkanes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
80
All alkenes show cis-trans isomerism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck








