Deck 6: Chemical Nomenclature
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/43
العب
ملء الشاشة (f)
Deck 6: Chemical Nomenclature
1
Examine the model of a form of naturally occurring sulfur. 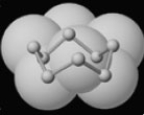 How should the formula for this form of sulfur be written?
How should the formula for this form of sulfur be written?
A)S
B)S4
C)S8
D)S16
E)S2
 How should the formula for this form of sulfur be written?
How should the formula for this form of sulfur be written?A)S
B)S4
C)S8
D)S16
E)S2
S8
2
Which of the following is the common name for the compound H2O?
A)Dihydrogen oxide
B)Dihydrogen monoxide
C)Hydrogen hydroxide
D)Monohydrogen hydroxide
E)None of the choices is correct
A)Dihydrogen oxide
B)Dihydrogen monoxide
C)Hydrogen hydroxide
D)Monohydrogen hydroxide
E)None of the choices is correct
None of the choices is correct
3
For each of the following names,choose the correct formula.
I)Oxide ion
Ii)Silver ion
Iii)Manganese(II)ion
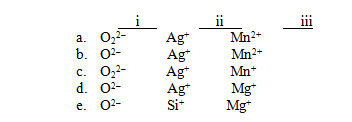
I)Oxide ion
Ii)Silver ion
Iii)Manganese(II)ion
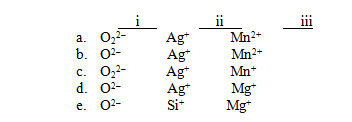
B
4
For each of the following formulas,choose the correct name.
(i)H2PO4-
(ii)HSO3-
(iii)HCO3-
A)(i)dihydrogen phosphate ion (ii)hydrogen sulfite ion (iii)hydrogen carbonate ion
B)(i)bihydrogen phosphate ion (ii)hydrogen sulfite ion (iii)hydrogen carbonate ion
C)(i)dihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrogen carbonate ion
D)(i)bihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrogen carbonate ion
E)(i)dihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrocarbonic ion
(i)H2PO4-
(ii)HSO3-
(iii)HCO3-
A)(i)dihydrogen phosphate ion (ii)hydrogen sulfite ion (iii)hydrogen carbonate ion
B)(i)bihydrogen phosphate ion (ii)hydrogen sulfite ion (iii)hydrogen carbonate ion
C)(i)dihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrogen carbonate ion
D)(i)bihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrogen carbonate ion
E)(i)dihydrogen phosphate ion (ii)hydrogen sulfate ion (iii)hydrocarbonic ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
5
Examine the name given on the following laboratory reagent container.The formula has been washed off the container.  What formula should be placed on the container (?)and what is the formula of the anion formed by this acid's total ionization in water,respectively?
What formula should be placed on the container (?)and what is the formula of the anion formed by this acid's total ionization in water,respectively?
A)H2SO3,SO32-
B)H2S,S2-
C)H2SO4,SO42-
D)H2SO4,HSO4-
E)H2SO2,SO22-
 What formula should be placed on the container (?)and what is the formula of the anion formed by this acid's total ionization in water,respectively?
What formula should be placed on the container (?)and what is the formula of the anion formed by this acid's total ionization in water,respectively?A)H2SO3,SO32-
B)H2S,S2-
C)H2SO4,SO42-
D)H2SO4,HSO4-
E)H2SO2,SO22-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements is false?
A)The first word in the name of a binary molecular compound is the name of the element appearing first in the chemical formula,including a prefix to indicate the number of atoms of that element in the molecule
B)The same two nonmetals often form more than one binary compound
C)If an element has no prefix in the name of a binary molecular compound,you may assume that there is only one atom of that element in the molecule
D)A compound whose formula ends in O5 is a pentaoxide rather than a pentoxide
E)The numerical prefix used in chemical names for the number 2 is di-.
A)The first word in the name of a binary molecular compound is the name of the element appearing first in the chemical formula,including a prefix to indicate the number of atoms of that element in the molecule
B)The same two nonmetals often form more than one binary compound
C)If an element has no prefix in the name of a binary molecular compound,you may assume that there is only one atom of that element in the molecule
D)A compound whose formula ends in O5 is a pentaoxide rather than a pentoxide
E)The numerical prefix used in chemical names for the number 2 is di-.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
7
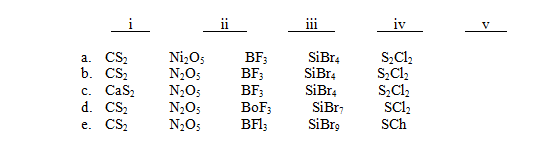
For each of the following names,choose the correct formula:
I)Carbon disulfide
Ii)Dinitrogen pentoxide
Iii)Boron trifluoride
Iv)Silicon tetrabromide
V)Disulfur dichloride
I)Carbon disulfide
Ii)Dinitrogen pentoxide
Iii)Boron trifluoride
Iv)Silicon tetrabromide
V)Disulfur dichloride


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following name-formula pairs is/are incorrect?
(i)Bromous acid,HBrO2
(ii)Nitrate ion,NO3-
(iii)Periodate ion,IO3-
(iv)Hydrofluoric acid,HFl
(v)Telluric acid,H2TeO3 (tellurium,Z = 52)
A)i and ii
B)iii and iv
C)iv and v
D)i,ii,and iii
E)iii,iv,and v
(i)Bromous acid,HBrO2
(ii)Nitrate ion,NO3-
(iii)Periodate ion,IO3-
(iv)Hydrofluoric acid,HFl
(v)Telluric acid,H2TeO3 (tellurium,Z = 52)
A)i and ii
B)iii and iv
C)iv and v
D)i,ii,and iii
E)iii,iv,and v

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
9
What are the formulas of water and ammonia,in that order?
A)H2O,NH4
B)H2O,NH4+
C)H2O,NH3
D)HO2,NH4
E)HO2,NH4+
A)H2O,NH4
B)H2O,NH4+
C)H2O,NH3
D)HO2,NH4
E)HO2,NH4+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following name-formula pairs is/are correct?
(i)Phosphoric acid / H3PO3
(ii)Sulfate ion / SO32-
(iii)Bromate ion / BrO3-
(iv)Hydrochloric acid / HCl
(v)Carbonate ion / CO3-
A)i and ii
B)iii and iv
C)ii,iii,and iv
D)i,ii,iii,and iv
E)all are correct
(i)Phosphoric acid / H3PO3
(ii)Sulfate ion / SO32-
(iii)Bromate ion / BrO3-
(iv)Hydrochloric acid / HCl
(v)Carbonate ion / CO3-
A)i and ii
B)iii and iv
C)ii,iii,and iv
D)i,ii,iii,and iv
E)all are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
11
For each of the following formulas,choose the correct name.
I)Pb2+
Ii)S2-
Iii)Ni2+

I)Pb2+
Ii)S2-
Iii)Ni2+


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
12
What are the names for the compounds H2O and NH3,in that order?
A)Dihydrogen oxide,nitrogen trihydride
B)Dihydrogen oxide,ammonia
C)Water,nitrogen trihydride
D)Water,ammonia
E)Hydrated oxygen,hydrated nitrogen
A)Dihydrogen oxide,nitrogen trihydride
B)Dihydrogen oxide,ammonia
C)Water,nitrogen trihydride
D)Water,ammonia
E)Hydrated oxygen,hydrated nitrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
13
For each of the following names,choose the correct formula.
I)Silicon tetrafluoride
Ii)Disulfur decafluoride
Iii)Sulfur trioxide
Iv)Diphosphorus pentoxide
V)Dichlorine oxide
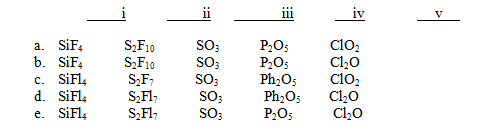
I)Silicon tetrafluoride
Ii)Disulfur decafluoride
Iii)Sulfur trioxide
Iv)Diphosphorus pentoxide
V)Dichlorine oxide


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
14
When nitrogen monoxide in the gas cylinder (1)reacts with air,nitrogen dioxide (2)is formed. 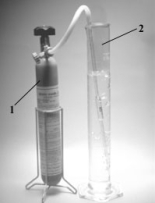 What are the formulas,respectively,for these two compounds?
What are the formulas,respectively,for these two compounds?
A)1 - N2O,2 - N2O2
B)1 - NO,2 - N2O
C)1 - NO,2 - NO2
D)1 - N2,2 - NO
E)1 - N2O2,2 - N2O4
 What are the formulas,respectively,for these two compounds?
What are the formulas,respectively,for these two compounds?A)1 - N2O,2 - N2O2
B)1 - NO,2 - N2O
C)1 - NO,2 - NO2
D)1 - N2,2 - NO
E)1 - N2O2,2 - N2O4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
15
For each of the following names,choose the correct formula.
I)Hydrogen sulfide ion
Ii)Hydrogen carbonate ion
Iii)Hydrogen phosphate ion
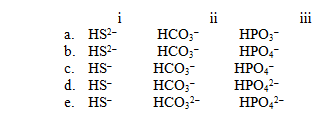
I)Hydrogen sulfide ion
Ii)Hydrogen carbonate ion
Iii)Hydrogen phosphate ion
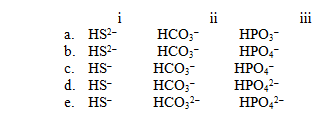

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
16
For each of the following names,choose the correct formula.
I)Mercury(II)ion
Ii)Nitride ion
Iii)Zinc ion
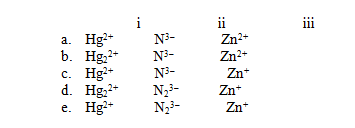
I)Mercury(II)ion
Ii)Nitride ion
Iii)Zinc ion
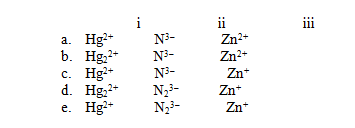

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following is the correct formula for ammonia?
A)NH3
B)NH4+
C)Hg22+
D)Hg2+
E)None of the choices is correct
A)NH3
B)NH4+
C)Hg22+
D)Hg2+
E)None of the choices is correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following name-formula pairs is/are correct?
(i)Sodium ion / Na+
(ii)Tin(II)ion / Ti2+
(iii)Silver ion / Ag+
(iv)Fluoride ion / Fl-
(v)Manganese(III)ion / Mn3+
A)i only
B)i and ii
C)i,iii,and v
D)i,iii,iv,and v
E)all are correct
(i)Sodium ion / Na+
(ii)Tin(II)ion / Ti2+
(iii)Silver ion / Ag+
(iv)Fluoride ion / Fl-
(v)Manganese(III)ion / Mn3+
A)i only
B)i and ii
C)i,iii,and v
D)i,iii,iv,and v
E)all are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following elemental formulas are written as they would appear in a chemical equation?
(i)Kr
(ii)Cl2
(iii)B
(iv)Be
(v)H2
A)ii and v
B)i and iii
C)iii and v
D)i,ii,iii,and v
E)i,ii,iii,iv,and v
(i)Kr
(ii)Cl2
(iii)B
(iv)Be
(v)H2
A)ii and v
B)i and iii
C)iii and v
D)i,ii,iii,and v
E)i,ii,iii,iv,and v

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
20
For each of the following names,choose the correct formula.
I)Sulfite ion
Ii)Phosphoric acid
Iii)Hydrosulfuric acid
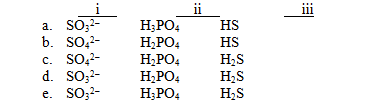
I)Sulfite ion
Ii)Phosphoric acid
Iii)Hydrosulfuric acid
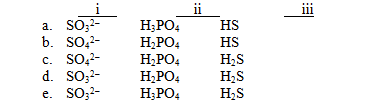

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
21
How many water molecules are associated with each formula unit of anhydrous cobalt perchlorate in Co(ClO4)2 • 6 H2O?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following name-formula pairs is/are correct?
(i)HSeO3-,hydrogen selenite ion (Z = 34)
(ii)HSO4-,hydrogen sulfite ion
(iii)H2PO4-,bihydrogen phosphate ion
(iv)HCO3-,hydrogen carbonate ion
A)i only
B)iii only
C)iv only
D)i and iii
E)i and iv
(i)HSeO3-,hydrogen selenite ion (Z = 34)
(ii)HSO4-,hydrogen sulfite ion
(iii)H2PO4-,bihydrogen phosphate ion
(iv)HCO3-,hydrogen carbonate ion
A)i only
B)iii only
C)iv only
D)i and iii
E)i and iv

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following is the correct formula for the ammonium ion?
A)NH4
B)NH4+
C)NH3+
D)NH3
E)NH2-
A)NH4
B)NH4+
C)NH3+
D)NH3
E)NH2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
24
The solution shown in the flask is being prepared by dissolving Ni(NO3)2 in water but the lab assistant has run out of this compound and needs to order more.  What name should be looked for in the catalog to order more of this compound?
What name should be looked for in the catalog to order more of this compound?
A)nickel nitride
B)nickel(II)nitrate
C)nickel nitrate
D)nickel nitrite
E)nickel(II)nitrogen oxide
 What name should be looked for in the catalog to order more of this compound?
What name should be looked for in the catalog to order more of this compound?A)nickel nitride
B)nickel(II)nitrate
C)nickel nitrate
D)nickel nitrite
E)nickel(II)nitrogen oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
25
What are the names of the compounds Hg2Cl2 and Zn3(PO4)2?
A)Mercury(II)chloride,zinc phosphate
B)Mercury(II)chloride,zinc(II)phosphate
C)Mercury(II)chloride,zinc(III)phosphate
D)Mercury(I)chloride,zinc phosphate
E)Mercury(I)chloride,zinc(II)phosphate
A)Mercury(II)chloride,zinc phosphate
B)Mercury(II)chloride,zinc(II)phosphate
C)Mercury(II)chloride,zinc(III)phosphate
D)Mercury(I)chloride,zinc phosphate
E)Mercury(I)chloride,zinc(II)phosphate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
26
What are the formulas of the compounds magnesium sulfate and lithium bromite?
A)Mg3(SO3)2,LiBr
B)MgSO3,LiBrO3
C)MgSO3,Li2BrO3
D)MgSO4,LiBrO2
E)MgSO4,Li2BrO3
A)Mg3(SO3)2,LiBr
B)MgSO3,LiBrO3
C)MgSO3,Li2BrO3
D)MgSO4,LiBrO2
E)MgSO4,Li2BrO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
27
What are the names of the compounds ZnCO3 and LiNO2?
A)Zinc carbonate,lithium nitrite
B)Zinc carbonate,lithium nitrate
C)Zinc(II)carbonate,lithium nitrite
D)Zinc(II)carbonate,lithium nitrate
E)Zinc(II)carbonate,monolithium nitrite
A)Zinc carbonate,lithium nitrite
B)Zinc carbonate,lithium nitrate
C)Zinc(II)carbonate,lithium nitrite
D)Zinc(II)carbonate,lithium nitrate
E)Zinc(II)carbonate,monolithium nitrite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the name of Cu(NO3)2 • 3 H2O?
A)Copper nitrate terthydrate
B)Copper(II)nitrate trihydrate
C)Copper nitrate terhydrate
D)Copper(II)nitrate terhydrate
E)Copper nitrate trihydrate
A)Copper nitrate terthydrate
B)Copper(II)nitrate trihydrate
C)Copper nitrate terhydrate
D)Copper(II)nitrate terhydrate
E)Copper nitrate trihydrate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
29
What are the names for the species OH- and NH4+?
A)Oxyhydrogen ion,nitroquadrahydrogen ion
B)Oxyhydrogen ion,ammonium ion
C)Oxyhydrogen ion,ammonia ion
D)Hydroxide ion,ammonium ion
E)Hydroxide ion,ammonia ion
A)Oxyhydrogen ion,nitroquadrahydrogen ion
B)Oxyhydrogen ion,ammonium ion
C)Oxyhydrogen ion,ammonia ion
D)Hydroxide ion,ammonium ion
E)Hydroxide ion,ammonia ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following is the correct formula for the hydroxide ion?
A)HO2-
B)HO2+
C)OH-
D)OH+
E)H3O+
A)HO2-
B)HO2+
C)OH-
D)OH+
E)H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
31
What are the formulas of the ammonium ion and the hydroxide ion,in that order?
A)NH4+,OH-
B)NH4+,OH+
C)NH4+,H3O+
D)NH3,OH-
E)NH3,HO2-
A)NH4+,OH-
B)NH4+,OH+
C)NH4+,H3O+
D)NH3,OH-
E)NH3,HO2-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
32
What are the formulas of the compounds calcium periodate and potassium nitrite?
A)Ca(IO4)2,KNO2
B)Ca(IO3)2,KNO2
C)Ca(IO4)2,KNO3
D)Ca(IO3)2,KNO3
E)CaIO4,KNO3
A)Ca(IO4)2,KNO2
B)Ca(IO3)2,KNO2
C)Ca(IO4)2,KNO3
D)Ca(IO3)2,KNO3
E)CaIO4,KNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following name-formula pairs is/are incorrect?
(i)HCO3- / hydrogen carbonate ion
(ii)HTe- / hydrogen telluride ion (Z = 52)
(iii)H2PO4- / hydrogen phosphate ion
(iv)HSO3- / hydrogen sulfite ion
A)iii only
B)i and ii
C)ii and iii
D)ii and iv
E)ii,iii,and iv
(i)HCO3- / hydrogen carbonate ion
(ii)HTe- / hydrogen telluride ion (Z = 52)
(iii)H2PO4- / hydrogen phosphate ion
(iv)HSO3- / hydrogen sulfite ion
A)iii only
B)i and ii
C)ii and iii
D)ii and iv
E)ii,iii,and iv

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
34
What are the formulas of the compounds zinc phosphide and potassium hydrogen phosphate?
A)Zn2P3,K2HPO4
B)Zn3P2,K2HPO4
C)Zn2P3,KHPO4
D)Zn3P2,KHPO4
E)Zn2P3,KH2PO4
A)Zn2P3,K2HPO4
B)Zn3P2,K2HPO4
C)Zn2P3,KHPO4
D)Zn3P2,KHPO4
E)Zn2P3,KH2PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many water molecules are associated with one formula unit of manganese(II)chloride in
MnCl2 • 4 H2O?
A)1
B)2
C)3
D)4
E)5
MnCl2 • 4 H2O?
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following formula-name pairs is/are correct?
(i)GaCl2,gallium chloride (gallium,Z = 31)
(ii)HS,hydrosulfuric acid
(iii)Pb(NO2)2,lead(II)nitrate
(iv)NaClO2,sodium chlorate
(v)K3PO4,potassium phosphate
A)all are correct
B)i,iii,and v
C)v only
D)i,ii,iii,and iv
E)none is correct
(i)GaCl2,gallium chloride (gallium,Z = 31)
(ii)HS,hydrosulfuric acid
(iii)Pb(NO2)2,lead(II)nitrate
(iv)NaClO2,sodium chlorate
(v)K3PO4,potassium phosphate
A)all are correct
B)i,iii,and v
C)v only
D)i,ii,iii,and iv
E)none is correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
37
What are the formulas of the compounds cobalt(III)sulfate and manganese(II)hydroxide?
A)Co2(SO4)3,MgOH2
B)Co2(SO4)4,Mg(OH)2
C)Co2(SO4)3,Mn(OH)2
D)Co2(SO4)2,Mn2OH
E)CoSO4,Mn(OH)2
A)Co2(SO4)3,MgOH2
B)Co2(SO4)4,Mg(OH)2
C)Co2(SO4)3,Mn(OH)2
D)Co2(SO4)2,Mn2OH
E)CoSO4,Mn(OH)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
38
What are the formulas of the compounds aluminum sulfide and ammonium chlorate?
A)AlS,NH3ClO4
B)Al3S2,NH3ClO3
C)Al2S3,NH4ClO4
D)Al2S3,(NH4)2ClO3
E)Al2S3,NH4ClO3
A)AlS,NH3ClO4
B)Al3S2,NH3ClO3
C)Al2S3,NH4ClO4
D)Al2S3,(NH4)2ClO3
E)Al2S3,NH4ClO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
39
Gypsum or calcium sulfate dihydrate is shown in the image.  What would be the formula for the anhydrous form of the compound produced when it is strongly heated?
What would be the formula for the anhydrous form of the compound produced when it is strongly heated?
A)CaSO4 • 2 H2O
B)CaSO4 • H2O
C)CaS • 2 H2O
D)CaS
E)CaSO4
 What would be the formula for the anhydrous form of the compound produced when it is strongly heated?
What would be the formula for the anhydrous form of the compound produced when it is strongly heated?A)CaSO4 • 2 H2O
B)CaSO4 • H2O
C)CaS • 2 H2O
D)CaS
E)CaSO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
40
What are the names of the compounds Ni(HCO3)2 and CrCl2?
A)nitrogen hydrogen carbonate,chromic chloride
B)nitrogen hydrogen carbonate,copper(II)chloride
C)nickel hydrogen carbonate,copper chloride
D)nickel(II)hydrogen carbonate,chromium(II)chloride
E)nickel hydrogen carbonate,chromium chloride
A)nitrogen hydrogen carbonate,chromic chloride
B)nitrogen hydrogen carbonate,copper(II)chloride
C)nickel hydrogen carbonate,copper chloride
D)nickel(II)hydrogen carbonate,chromium(II)chloride
E)nickel hydrogen carbonate,chromium chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the name of Fe(NO3)3 • 9 H2O?
A)Iron(III)nitrate ninhydrate
B)Iron(III)nitrate nonahydrate
C)Iron(III)nitrate heptahydrate
D)Iron(III)nitrite nonahydrate
E)Iron(III)nitrite heptahydrate
A)Iron(III)nitrate ninhydrate
B)Iron(III)nitrate nonahydrate
C)Iron(III)nitrate heptahydrate
D)Iron(III)nitrite nonahydrate
E)Iron(III)nitrite heptahydrate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the formula of cobalt(II)nitrate hexahydrate?
A)[Co(NO3)2]6(H2O)
B)[Co2NO3]6(H2O)
C)Co(NO3)2 • 6 H2O
D)Cb(NO3)2 • 6 H2O
E)C2NO3 • 6 H2O
A)[Co(NO3)2]6(H2O)
B)[Co2NO3]6(H2O)
C)Co(NO3)2 • 6 H2O
D)Cb(NO3)2 • 6 H2O
E)C2NO3 • 6 H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the formula of barium hydroxide octahydrate?
A)Ba(OH)2 • 8 H2O
B)BaOH • 8 H2O
C)B(OH)3 • 8 H2O
D)B(OH)2 • 8 H2O
E)B(OH)3 + 8 H2O( )
)
A)Ba(OH)2 • 8 H2O
B)BaOH • 8 H2O
C)B(OH)3 • 8 H2O
D)B(OH)2 • 8 H2O
E)B(OH)3 + 8 H2O(
 )
)
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 43 في هذه المجموعة.
فتح الحزمة
k this deck








